Innovative Therapy for Alzheimer's Disease-With Focus on Biodelivery of NGF
- PMID: 30804738
- PMCID: PMC6370742
- DOI: 10.3389/fnins.2019.00038
Innovative Therapy for Alzheimer's Disease-With Focus on Biodelivery of NGF
Abstract
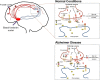
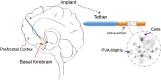
Alzheimer's disease (AD) is a progressive neurodegenerative disorder associated with abnormal protein modification, inflammation and memory impairment. Aggregated amyloid beta (Aβ) and phosphorylated tau proteins are medical diagnostic features. Loss of memory in AD has been associated with central cholinergic dysfunction in basal forebrain, from where the cholinergic circuitry projects to cerebral cortex and hippocampus. Various reports link AD progression with declining activity of cholinergic neurons in basal forebrain. The neurotrophic molecule, nerve growth factor (NGF), plays a major role in the maintenance of cholinergic neurons integrity and function, both during development and adulthood. Numerous studies have also shown that NGF contributes to the survival and regeneration of neurons during aging and in age-related diseases such as AD. Changes in neurotrophic signaling pathways are involved in the aging process and contribute to cholinergic and cognitive decline as observed in AD. Further, gradual dysregulation of neurotrophic factors like NGF and brain derived neurotrophic factor (BDNF) have been reported during AD development thus intensifying further research in targeting these factors as disease modifying therapies against AD. Today, there is no cure available for AD and the effects of the symptomatic treatment like cholinesterase inhibitors (ChEIs) and memantine are transient and moderate. Although many AD treatment studies are being carried out, there has not been any breakthrough and new therapies are thus highly needed. Long-term effective therapy for alleviating cognitive impairment is a major unmet need. Discussion and summarizing the new advancements of using NGF as a potential therapeutic implication in AD are important. In summary, the intent of this review is describing available experimental and clinical data related to AD therapy, priming to gain additional facts associated with the importance of NGF for AD treatment, and encapsulated cell biodelivery (ECB) as an efficient tool for NGF delivery.
Keywords: Alzheimer disease; NGF; cholinergic; encapsulated cell biodelivery; neurotrophins.
Figures
References
-
- Ali Shariati M., Kumar V., Yang T., Chakraborty C., Barres B. A., Longo F. M., et al. (2018). A small molecule TrkB neurotrophin receptor partial agonist as possible treatment for experimental nonarteritic anterior ischemic optic neuropathy. Curr. Eye Res. 43 1489–1499. 10.1080/02713683.2018.1508726 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

