Randomized Controlled Trial of Physical Exercise in Diabetic Veterans With Length-Dependent Distal Symmetric Polyneuropathy
- PMID: 30804739
- PMCID: PMC6379046
- DOI: 10.3389/fnins.2019.00051
Randomized Controlled Trial of Physical Exercise in Diabetic Veterans With Length-Dependent Distal Symmetric Polyneuropathy
Abstract
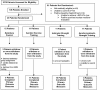
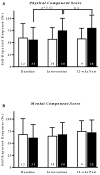
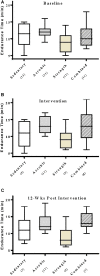
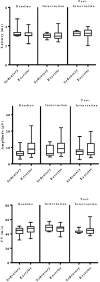
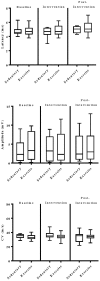
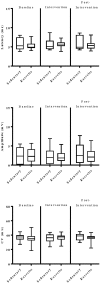
Rationale: Physical exercise is an essential adjunct to the management of patients with type 2 diabetes mellitus. Therapeutic interventions that improve blood flow to peripheral nerves, such as exercise, may slow the progression of neuropathy in the diabetic patient. Aims: This randomized clinical trial was conducted to determine whether a structured program of aerobic, isokinetic strength, or the combination of aerobic-isokinetic strength exercise intervention alters peripheral nerve function in glycemic-controlled diabetic patients with advanced length-dependent distal symmetric polyneuropathy. Methods: Forty-five patients with type 2 diabetes mellitus exhibiting tight glycemic control (HbA1c intergroup range 7.2-8.0%) were randomized by block design across four experimental groups: sedentary controls (n = 12), aerobic exercise (n = 11), isokinetic strength (n = 11), or the combination of aerobic-isokinetic strength training (n = 11). Patients randomized to training groups exercised 3× per week for 12 weeks, whereas patients randomized to the sedentary control group received standard of care. To minimize attention and educational bias, all patients attended a 12-session health promotion educational series. At baseline, immediately following intervention, and again at 12-week post-intervention, detailed nerve conduction studies were conducted as a primary outcome measure. At these same intervals, all patients completed as secondary measures quantitative sensory testing, symptom-limited treadmill stress tests, and a Short-Form 36-Veterans Questionnaire (SF-36V). Results: Of the 45 patients randomized into this study, 37 (82%) had absent sural nerve responses, 19 (42%) had absent median sensory nerve responses, and 17 (38%) had absent ulnar sensory nerve responses. By comparison, responses from tibial nerves were absent in only three (7%) subjects while responses from peroneal nerves were absent in five (11%) subjects. Eleven (92%) of 12 patients that had volunteered to be biopsied exhibited abnormal levels of epidermal nerve fiber densities. Exercise, regardless of type, did not alter sensory or motor nerve electrodiagnostic findings among those patients exhibiting measurable responses (ANOVA). There was, however, a modest (p = 0.01) beneficial effect of exercise on sensory nerve function (Fisher's Exact Test). Importantly, the beneficial effect of exercise on sensory nerve function was enhanced (p = 0.03) during the post-intervention interval. In addition, three of six patients that had undergone exercise intervention exhibited a marked 1.9 ± 0.3-fold improvement in epidermal nerve fiber density. By comparison, none of three sedentary patients whom agreed to be biopsied a second time showed improvement in epidermal nerve fiber density. Compared to baseline values within groups, and compared with sedentary values across groups, neither aerobic, isokinetic strength, or the combination of aerobic-isokinetic strength exercise intervention altered peak oxygen uptake. Patients that underwent aerobic or the combined aerobic-isokinetic strength exercise intervention, however, demonstrated an increase in treadmill test duration that was sustained over the 12-week post-intervention period. Conclusion: A 12-week course of physical exercise, regardless of type, does not alter sensory or motor nerve electrodiagnostic findings. In a subset of patients, a short-term structured program of aerobic exercise may selectively improve sensory nerve fiber function. Large-scale exercise lifestyle intervention trials are warranted to further evaluate the impact of aerobic exercise on sensory nerve fiber function in diabetic neuropathic patients. Clinical Trial Registration: www.ClinicalTrials.gov, identifier NCT00955201.
Keywords: diabetes; exercise; human; metabolic; nerve conduction studies; peripheral nerve.
Figures


Similar articles
-
The Effects of a 10-Week Aerobic and Unilateral Lower Extremity Resistance Training Program on Amplitude and Nerve Conduction Velocity of Sensory and Motor Nerves in Diabetic Patients with Neuropathy.J Hum Kinet. 2023 Apr 20;87:93-103. doi: 10.5114/jhk/161610. eCollection 2023 Apr. J Hum Kinet. 2023. PMID: 37229418 Free PMC article.
-
Erratum.Mult Scler. 2016 Oct;22(12):NP9-NP11. doi: 10.1177/1352458515585718. Epub 2015 Jun 3. Mult Scler. 2016. PMID: 26041800
-
Effect of aerobic training on nerve conduction in men with type 2 diabetes and peripheral neuropathy: A randomized controlled trial.Neurophysiol Clin. 2018 Sep;48(4):195-202. doi: 10.1016/j.neucli.2018.03.001. Epub 2018 Mar 30. Neurophysiol Clin. 2018. PMID: 29606547 Clinical Trial.
-
The Effectiveness of Aerobic Exercise in Improving Peripheral Nerve Functions in Type 2 Diabetes Mellitus: An Evidence Based Case Report.Acta Med Indones. 2018 Jan;50(1):82-87. Acta Med Indones. 2018. PMID: 29686181 Review.
-
Impact of Exercise Training in Patients with Diabetic Peripheral Neuropathy: An Umbrella Review.Sports Med Open. 2025 Jun 15;11(1):75. doi: 10.1186/s40798-025-00863-4. Sports Med Open. 2025. PMID: 40517337 Free PMC article.
Cited by
-
Exercise and Neuropathic Pain: A General Overview of Preclinical and Clinical Research.Sports Med Open. 2021 Mar 22;7(1):21. doi: 10.1186/s40798-021-00307-9. Sports Med Open. 2021. PMID: 33751253 Free PMC article. Review.
-
Association Between Physical Exercise Interventions Participation and Functional Capacity in Individuals with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Controlled Trials.Sports Med Open. 2022 Mar 4;8(1):34. doi: 10.1186/s40798-022-00422-1. Sports Med Open. 2022. PMID: 35244804 Free PMC article.
-
The global and regional burden of diabetic peripheral neuropathy.Nat Rev Neurol. 2025 Jan;21(1):17-31. doi: 10.1038/s41582-024-01041-y. Epub 2024 Dec 5. Nat Rev Neurol. 2025. PMID: 39639140 Review.
-
Issues and challenges in diabetic neuropathy management: A narrative review.World J Diabetes. 2023 Jun 15;14(6):741-757. doi: 10.4239/wjd.v14.i6.741. World J Diabetes. 2023. PMID: 37383599 Free PMC article. Review.
-
The Effects of a 10-Week Aerobic and Unilateral Lower Extremity Resistance Training Program on Amplitude and Nerve Conduction Velocity of Sensory and Motor Nerves in Diabetic Patients with Neuropathy.J Hum Kinet. 2023 Apr 20;87:93-103. doi: 10.5114/jhk/161610. eCollection 2023 Apr. J Hum Kinet. 2023. PMID: 37229418 Free PMC article.
References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

