Bumetanide Prevents Brain Trauma-Induced Depressive-Like Behavior
- PMID: 30804751
- PMCID: PMC6370740
- DOI: 10.3389/fnmol.2019.00012
Bumetanide Prevents Brain Trauma-Induced Depressive-Like Behavior
Abstract
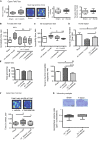
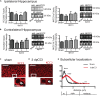
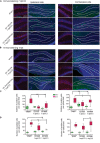
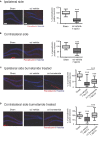
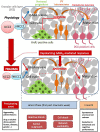
Brain trauma triggers a cascade of deleterious events leading to enhanced incidence of drug resistant epilepsies, depression, and cognitive dysfunctions. The underlying mechanisms leading to these alterations are poorly understood and treatment that attenuates those sequels are not available. Using controlled-cortical impact as an experimental model of brain trauma in adult mice, we found a strong suppressive effect of the sodium-potassium-chloride importer (NKCC1) specific antagonist bumetanide on the appearance of depressive-like behavior. We demonstrate that this alteration in behavior is associated with an impairment of post-traumatic secondary neurogenesis within the dentate gyrus of the hippocampus. The mechanism mediating the effect of bumetanide involves early transient changes in the expression of chloride regulatory proteins and qualitative changes in GABA(A) mediated transmission from hyperpolarizing to depolarizing after brain trauma. This work opens new perspectives in the early treatment of human post-traumatic induced depression. Our results strongly suggest that bumetanide might constitute an efficient prophylactic treatment to reduce neurological and psychiatric consequences of brain trauma.
Keywords: bumetanide; depression; interneuron cell death; neurogenesis; potassium chloride cotransporter 2 (KCC2); psychiatric disease.
Figures

References
-
- Amini-Khoei H., Mohammadi-Asl A., Amiri S., Hosseini M.-J., Momeny M., Hassanipour M., et al. (2017). Oxytocin mitigated the depressive-like behaviors of maternal separation stress through modulating mitochondrial function and neuroinflammation. Prog. Neuropsychopharmacol. Biol. Psychiatry 76 169–178. 10.1016/j.pnpbp.2017.02.022 - DOI - PubMed
LinkOut - more resources
Full Text Sources

