Combination Therapy With Fingolimod and Neural Stem Cells Promotes Functional Myelination in vivo Through a Non-immunomodulatory Mechanism
- PMID: 30804753
- PMCID: PMC6371042
- DOI: 10.3389/fncel.2019.00014
Combination Therapy With Fingolimod and Neural Stem Cells Promotes Functional Myelination in vivo Through a Non-immunomodulatory Mechanism
Erratum in
-
Corrigendum: Combination therapy with fingolimod and neural stem cells promotes functional myelination in vivo through a non-immunomodulatory mechanism.Front Cell Neurosci. 2022 Dec 15;16:1022297. doi: 10.3389/fncel.2022.1022297. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36589285 Free PMC article.
Abstract
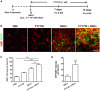
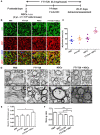
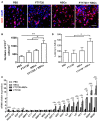
Myelination, which occurs predominantly postnatally and continues throughout life, is important for proper neurologic function of the mammalian central nervous system (CNS). We have previously demonstrated that the combination therapy of fingolimod (FTY720) and transplanted neural stem cells (NSCs) had a significantly enhanced therapeutic effect on the chronic stage of experimental autoimmune encephalomyelitis, an animal model of CNS autoimmunity, compared to using either one of them alone. However, reduced disease severity may be secondary to the immunomodulatory effects of FTY720 and NSCs, while whether this therapy directly affects myelinogenesis remains unknown. To investigate this important question, we used three myelination models under minimal or non-inflammatory microenvironments. Our results showed that FTY720 drives NSCs to differentiate into oligodendrocytes and promotes myelination in an ex vivo brain slice culture model, and in the developing CNS of healthy postnatal mice in vivo. Elevated levels of neurotrophic factors, e.g., brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor, were observed in the CNS of the treated infant mice. Further, FTY720 and NSCs efficiently prolonged the survival and improved sensorimotor function of shiverer mice. Together, these data demonstrate a direct effect of FTY720, beyond its known immunomodulatory capacity, in NSC differentiation and myelin development as a novel mechanism underlying its therapeutic effect in demyelinating diseases.
Keywords: combination therapy; fingolimod; myelination; neural stem cells; oligodendrocytes.
Figures



Similar articles
-
Effect of Fingolimod on Neural Stem Cells: A Novel Mechanism and Broadened Application for Neural Repair.Mol Ther. 2017 Feb 1;25(2):401-415. doi: 10.1016/j.ymthe.2016.12.008. Epub 2016 Dec 28. Mol Ther. 2017. PMID: 28153091 Free PMC article.
-
Neural stem cells derived from primitive mesenchymal stem cells reversed disease symptoms and promoted neurogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis.Stem Cell Res Ther. 2021 Sep 9;12(1):499. doi: 10.1186/s13287-021-02563-8. Stem Cell Res Ther. 2021. PMID: 34503569 Free PMC article.
-
Effects of FTY720 on brain neurogenic niches in vitro and after kainic acid-induced injury.J Neuroinflammation. 2017 Jul 24;14(1):147. doi: 10.1186/s12974-017-0922-6. J Neuroinflammation. 2017. PMID: 28738875 Free PMC article.
-
Neural Stem Cell-Based Regenerative Approaches for the Treatment of Multiple Sclerosis.Mol Neurobiol. 2018 Apr;55(4):3152-3171. doi: 10.1007/s12035-017-0566-7. Epub 2017 May 2. Mol Neurobiol. 2018. PMID: 28466274 Free PMC article. Review.
-
Central nervous system-directed effects of FTY720 (fingolimod).J Neurol Sci. 2008 Nov 15;274(1-2):13-7. doi: 10.1016/j.jns.2008.06.031. Epub 2008 Aug 3. J Neurol Sci. 2008. PMID: 18678377 Review.
Cited by
-
Clinical Potential of Immunotherapies in Subarachnoid Hemorrhage Treatment: Mechanistic Dissection of Innate and Adaptive Immune Responses.Aging Dis. 2023 Oct 1;14(5):1533-1554. doi: 10.14336/AD.2023.0126. Aging Dis. 2023. PMID: 37196120 Free PMC article. Review.
-
Nerve-Glial antigen 2: unmasking the enigmatic cellular identity in the central nervous system.Front Immunol. 2024 Jul 29;15:1393842. doi: 10.3389/fimmu.2024.1393842. eCollection 2024. Front Immunol. 2024. PMID: 39136008 Free PMC article. Review.
-
Signaling through the S1P-S1PR Axis in the Gut, the Immune and the Central Nervous System in Multiple Sclerosis: Implication for Pathogenesis and Treatment.Cells. 2021 Nov 18;10(11):3217. doi: 10.3390/cells10113217. Cells. 2021. PMID: 34831439 Free PMC article. Review.
-
Generation of Oligodendrocyte Progenitor Cells From Mouse Bone Marrow Cells.Front Cell Neurosci. 2019 Jun 5;13:247. doi: 10.3389/fncel.2019.00247. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31231194 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

