Smilagenin Protects Dopaminergic Neurons in Chronic MPTP/Probenecid-Lesioned Parkinson's Disease Models
- PMID: 30804756
- PMCID: PMC6371654
- DOI: 10.3389/fncel.2019.00018
Smilagenin Protects Dopaminergic Neurons in Chronic MPTP/Probenecid-Lesioned Parkinson's Disease Models
Abstract
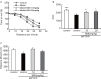
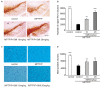
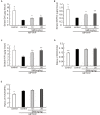
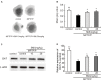
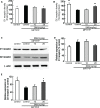
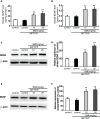
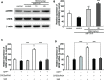
Current therapies for Parkinson's disease (PD) only offer limited symptomatic alleviation but fail to hamper the progress of the disease. Thus, it is imperative to establish new approaches aiming at protecting or reversing neurodegeneration in PD. Recent work elucidates whether smilagenin (abbreviated SMI), a steroidal sapogenin from traditional Chinese medicinal herbs, can take neuroprotective effect on dopaminergic neurons in a chronic model of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) conjuncted with probenecid mice. We reported for the first time that SMI significantly improved the locomotor ability of chronic MPTP/probenecid-lesioned mice. SMI increased the tyrosine hydroxylase (TH) positive and Nissl positive neuron number in the substantia nigra pars compacta (SNpc), augmented striatal DA and its metabolites concentration and elevated striatal dopamine transporter density (DAT). In addition, dopamine receptor D2R not D1R was down-regulated by MPTP/probenecid and slightly raised by SMI prevention. What's more, we discovered that SMI markedly elevated striatal glial cell line-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) protein levels in SMI prevented mice. And we found that SMI increased GDNF and BDNF mRNA level by promoting CREB phosphorylation in 1-methyl-4-phenylpyridimium (MPP+) treated SH-SY5Y cells. The results illustrated that SMI could prevent the impairment of dopaminergic neurons in chronic MPTP/probenecid-induced mouse model.
Keywords: Chinese herb; Parkinson’s disease; brain-derived neurotrophic factor; dopaminergic neurons; glial cell line-derived neurotrophic factor.
Figures
Similar articles
-
Catalpol attenuates MPTP induced neuronal degeneration of nigral-striatal dopaminergic pathway in mice through elevating glial cell derived neurotrophic factor in striatum.Neuroscience. 2010 Apr 28;167(1):174-84. doi: 10.1016/j.neuroscience.2010.01.048. Epub 2010 Feb 1. Neuroscience. 2010. PMID: 20123001
-
Harpagoside attenuates MPTP/MPP⁺ induced dopaminergic neurodegeneration and movement disorder via elevating glial cell line-derived neurotrophic factor.J Neurochem. 2012 Mar;120(6):1072-83. doi: 10.1111/j.1471-4159.2011.07635.x. Epub 2012 Feb 6. J Neurochem. 2012. PMID: 22192054
-
Telmisartan attenuates MPTP induced dopaminergic degeneration and motor dysfunction through regulation of α-synuclein and neurotrophic factors (BDNF and GDNF) expression in C57BL/6J mice.Neuropharmacology. 2013 Oct;73:98-110. doi: 10.1016/j.neuropharm.2013.05.025. Epub 2013 Jun 6. Neuropharmacology. 2013. PMID: 23747572
-
Epigenetic targeting of histone deacetylase: therapeutic potential in Parkinson's disease?Pharmacol Ther. 2013 Oct;140(1):34-52. doi: 10.1016/j.pharmthera.2013.05.010. Epub 2013 May 24. Pharmacol Ther. 2013. PMID: 23711791 Review.
-
Dynamic Changes in the Nigrostriatal Pathway in the MPTP Mouse Model of Parkinson's Disease.Parkinsons Dis. 2017;2017:9349487. doi: 10.1155/2017/9349487. Epub 2017 Jul 31. Parkinsons Dis. 2017. PMID: 28831326 Free PMC article. Review.
Cited by
-
Protective effect of Tongdu Tiaoshen acupuncture combined with Xiaoxuming decoction on dopaminergic neurons in Parkinson's disease model.J Tradit Chin Med. 2023 Jun;43(3):484-493. doi: 10.19852/j.cnki.jtcm.20230214.005. J Tradit Chin Med. 2023. PMID: 37147749 Free PMC article.
-
Restoration of Noradrenergic Function in Parkinson's Disease Model Mice.ASN Neuro. 2021 Jan-Dec;13:17590914211009730. doi: 10.1177/17590914211009730. ASN Neuro. 2021. PMID: 33940943 Free PMC article.
-
Er-Bai-Tang decoction improved the movement disorders and neuronal injury in the Parkinson's disease model rats via decreasing p38 MAPK pathway and improving the composition of intestinal flora.Acta Cir Bras. 2023 Jan 6;37(11):e371104. doi: 10.1590/acb371104. eCollection 2023. Acta Cir Bras. 2023. PMID: 36629531 Free PMC article.
-
Nanoformulations of Herbal Extracts in Treatment of Neurodegenerative Disorders.Front Bioeng Biotechnol. 2020 Apr 7;8:238. doi: 10.3389/fbioe.2020.00238. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32318551 Free PMC article. Review.
-
BDNF as a Promising Therapeutic Agent in Parkinson's Disease.Int J Mol Sci. 2020 Feb 10;21(3):1170. doi: 10.3390/ijms21031170. Int J Mol Sci. 2020. PMID: 32050617 Free PMC article. Review.
References
-
- Anandhan A., Janakiraman U., Manivasagam T. (2012). Theaflavin ameliorates behavioral deficits, biochemical indices and monoamine transporters expression against subacute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mouse model of Parkinson’s disease. Neuroscience 218, 257–267. 10.1016/j.neuroscience.2012.05.039 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

