The Role of APOE4 in Disrupting the Homeostatic Functions of Astrocytes and Microglia in Aging and Alzheimer's Disease
- PMID: 30804776
- PMCID: PMC6378415
- DOI: 10.3389/fnagi.2019.00014
The Role of APOE4 in Disrupting the Homeostatic Functions of Astrocytes and Microglia in Aging and Alzheimer's Disease
Abstract
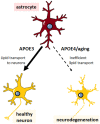
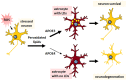
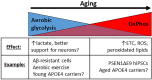
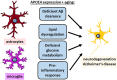
APOE4 is the greatest genetic risk factor for late-onset Alzheimer's disease (AD), increasing the risk of developing the disease by 3-fold in the 14% of the population that are carriers. Despite 25 years of research, the exact mechanisms underlying how APOE4 contributes to AD pathogenesis remain incompletely defined. APOE in the brain is primarily expressed by astrocytes and microglia, cell types that are now widely appreciated to play key roles in the pathogenesis of AD; thus, a picture is emerging wherein APOE4 disrupts normal glial cell biology, intersecting with changes that occur during normal aging to ultimately cause neurodegeneration and cognitive dysfunction. This review article will summarize how APOE4 alters specific pathways in astrocytes and microglia in the context of AD and the aging brain. APOE itself, as a secreted lipoprotein without enzymatic activity, may prove challenging to directly target therapeutically in the classical sense. Therefore, a deeper understanding of the underlying pathways responsible for APOE4 toxicity is needed so that more tractable pathways and drug targets can be identified to reduce APOE4-mediated disease risk.
Keywords: APOE; Alzheimer’s disease; aging; astrocytes; microglia.
Figures


References
-
- Abe-Dohmae S., Yokoyama S. (2012). ABCA7: a potential mediator between cholesterol homeostasis and the host defense system. Clin. Lipidol. 7, 677–687. 10.2217/clp.12.67 - DOI
-
- Akiyama T. E., Sakai S., Lambert G., Nicol C. J., Matsusue K., Pimprale S., et al. (2002). Conditional disruption of the peroxisome proliferator-activated receptor γ gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol. Cell. Biol. 22, 2607–2619. 10.1128/mcb.22.8.2607-2619.2002 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

