Ferulic Acid Protects Hyperglycemia-Induced Kidney Damage by Regulating Oxidative Insult, Inflammation and Autophagy
- PMID: 30804780
- PMCID: PMC6371841
- DOI: 10.3389/fphar.2019.00027
Ferulic Acid Protects Hyperglycemia-Induced Kidney Damage by Regulating Oxidative Insult, Inflammation and Autophagy
Abstract
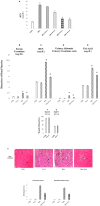
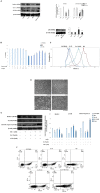
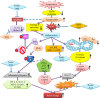
Oxidative insult, inflammation, apoptosis and autophagy play a pivotal role in the etiology of diabetic nephropathy, a global health concern. Ferulic acid, a phytochemical, is reported to protect against varied diseased conditions. However, the ameliorative role and mechanisms of ferulic acid in averting STZ-mediated nephrotoxicity largely remains unknown. For in vivo study, a single intraperitoneal injection of streptozotocin (50 mg kg-1 body wt.) was administered in experimental rats to induce diabetes. The diabetic rats exhibited a rise in blood glucose level as well as kidney to body weight ratio, a decrease in serum insulin level, severe kidney tissue damage and dysfunction. Elevation of intracellular ROS level, altered mitochondrial membrane potential and cellular redox balance impairment shown the participation of oxidative stress in hyperglycemia-triggered renal injury. Treatment with ferulic acid (50 mg kg-1 body wt., orally for 8 weeks), post-diabetic induction, could markedly ameliorate kidney injury, renal cell apoptosis, inflammation and defective autophagy in the kidneys. The underlying mechanism for such protection involved the modulation of AGEs, MAPKs (p38, JNK, and ERK 1/2), NF-κB mediated inflammatory pathways, mitochondria-dependent and -independent apoptosis as well as autophagy induction. In cultured NRK-52E cells, ferulic acid (at an optimum dose of 75 μM) could counter excessive ROS generation, induce autophagy and inhibit apoptotic death of cells under high glucose environment. Blockade of autophagy could significantly eradicate the protective effect of ferulic acid in high glucose-mediated cell death. Together, the study confirmed that ferulic acid, exhibiting hypoglycemic, antioxidant, anti-inflammatory, anti-apoptotic activities and role in autophagy, could circumvent oxidative stress-mediated renal cell damage.
Keywords: apoptosis; autophagy; diabetes; ferulic acid; inflammation; kidney; oxidative stress.
Figures

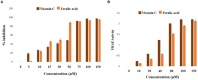






Similar articles
-
Deciphering the role of ferulic acid against streptozotocin-induced cellular stress in the cardiac tissue of diabetic rats.Food Chem Toxicol. 2016 Nov;97:187-198. doi: 10.1016/j.fct.2016.09.011. Epub 2016 Sep 9. Food Chem Toxicol. 2016. PMID: 27621051
-
Ferulic acid in the treatment of post-diabetes testicular damage: relevance to the down regulation of apoptosis correlates with antioxidant status via modulation of TGF-β1, IL-1β and Akt signalling.Cell Biochem Funct. 2014 Jan;32(1):115-24. doi: 10.1002/cbf.2983. Epub 2013 May 10. Cell Biochem Funct. 2014. PMID: 23661600
-
Ameliorative role of ferulic acid against diabetes associated oxidative stress induced spleen damage.Food Chem Toxicol. 2018 Aug;118:272-286. doi: 10.1016/j.fct.2018.05.029. Epub 2018 May 11. Food Chem Toxicol. 2018. PMID: 29758315
-
Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress.Life Sci. 2018 Jan 15;193:20-33. doi: 10.1016/j.lfs.2017.12.001. Epub 2017 Dec 5. Life Sci. 2018. PMID: 29203148 Review.
-
Molecular mechanism and research progress on pharmacology of ferulic acid in liver diseases.Front Pharmacol. 2023 May 31;14:1207999. doi: 10.3389/fphar.2023.1207999. eCollection 2023. Front Pharmacol. 2023. PMID: 37324465 Free PMC article. Review.
Cited by
-
Dietary ferulic acid supplementation improved cottonseed meal-based diet utilization by enhancing intestinal physical barrier function and liver antioxidant capacity in grass carp (Ctenopharyngodon Idellus).Front Physiol. 2022 Aug 22;13:922037. doi: 10.3389/fphys.2022.922037. eCollection 2022. Front Physiol. 2022. PMID: 36072855 Free PMC article.
-
Chinese Herbal Medicine in Ameliorating Diabetic Kidney Disease via Activating Autophagy.J Diabetes Res. 2019 Nov 16;2019:9030893. doi: 10.1155/2019/9030893. eCollection 2019. J Diabetes Res. 2019. PMID: 31828168 Free PMC article. Review.
-
Tangshenning Attenuates High Glucose-Induced Podocyte Injury via Restoring Autophagy Activity through Inhibiting mTORC1 Activation.J Diabetes Res. 2022 Jun 28;2022:1610416. doi: 10.1155/2022/1610416. eCollection 2022. J Diabetes Res. 2022. PMID: 35799948 Free PMC article.
-
Role of Bioactive Compounds in Obesity: Metabolic Mechanism Focused on Inflammation.Foods. 2022 Apr 25;11(9):1232. doi: 10.3390/foods11091232. Foods. 2022. PMID: 35563955 Free PMC article. Review.
-
Ferulic acid mediates prebiotic responses of cereal-derived arabinoxylans on host health.Anim Nutr. 2021 Oct 28;9:31-38. doi: 10.1016/j.aninu.2021.08.004. eCollection 2022 Jun. Anim Nutr. 2021. PMID: 35949987 Free PMC article. Review.
References
-
- Barr C. C. (2001). Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive insulin therapy, by The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N. Engl. J. Med 342: 381-9, 2000. Surv. Ophthalmol. 45 459–460. 10.1016/S0039-6257(01)00187-4 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

