Kininogen Level in the Cerebrospinal Fluid May Be a Potential Biomarker for Predicting Epileptogenesis
- PMID: 30804871
- PMCID: PMC6371036
- DOI: 10.3389/fneur.2019.00037
Kininogen Level in the Cerebrospinal Fluid May Be a Potential Biomarker for Predicting Epileptogenesis
Abstract
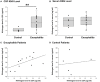
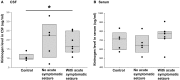
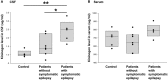
Purpose: Epilepsy is a highly disabling neurological disorder. Brain insult is the most critical cause of epilepsy in adults. This study aimed to find reliable and efficient biomarkers for predicting secondary epilepsy. Materials and methods: The LiCl-pilocarpine (LiCl-Pilo) chronic epilepsy rat model was used, and rat cerebrospinal fluid (CSF) was collected 5 days after status epilepticus (SE). The CSF was analyzed using the label-free LC-ESI-Q-TOF-MS/MS. Differential expression of proteins was confirmed using enzyme-linked immunosorbent assay (ELISA) and Western blotting. The corresponding protein level in the CSF of patients with encephalitis in the postacute phase was determined using ELISA and compared between patients with and without symptomatic epilepsy after encephalitis during a 2-year follow-up. Results: The proteomics and ELISA results showed that the protein level of kininogen (KNG) was obviously elevated in both CSF and hippocampus, but not in serum, 5 days after the onset of SE in LiCl-Pilo chronic epilepsy model rats. In patients with encephalitis, the protein level of KNG in the CSF in the postacute phase was significantly elevated in patients with a recurrent epileptic seizure during a 2-year follow-up than in patients without a recurrent seizure. Conclusion: KNG in the CSF may serve as a potential biomarker for predicting epileptogenesis in patients with encephalitis.
Keywords: biomarker; encephalitis; epilepsy; epileptogenesis; kininogen; pilocarpine; proteomics.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

