Neuro-Behavioral Status and the Hippocampal Expression of Metabolic Associated Genes in Wild-Type Rat Following a Ketogenic Diet
- PMID: 30804881
- PMCID: PMC6370680
- DOI: 10.3389/fneur.2019.00065
Neuro-Behavioral Status and the Hippocampal Expression of Metabolic Associated Genes in Wild-Type Rat Following a Ketogenic Diet
Abstract
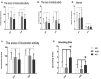
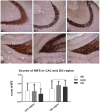
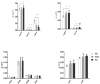
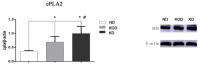
While a ketogenic diet (KD) is a well-established therapy for medically intractable epilepsy, clinical evidence of relevant adverse events of a KD has also been reported. We asked whether this kind of diet would have deleterious effects on wild-type brain function by evaluating KD-induced biochemical changes in the hippocampus as well as neurobehavioral changes occurring in wild-type rats. Fifty-four Sprague-Dawley rats were randomly assigned to three groups on postnatal day 28 (P28): wild-type rats fed with a KD qd (daily for 4 weeks, KD) or qod (every other day for 4 weeks, KOD), and wild-type rats fed with standard normal laboratory diet (ND). Neurobehavioral changes were observed on P35, P42, and P49. The hippocampal mossy fiber sprouting, the expression levels of zinc transporters (ZnTs) and lipid metabolism related genes were detected by Timm staining, RT-qPCR and western blot analysis, respectively, on P58. The KD-treated KOD and KD groups showed a significant delay of negative geotaxis reflex on P35, but not on P42 or P49. In the open field test, daily KD treatment only led to a reduction in exploratory activity and increased grooming times but induced no significant changes in the scores of vertical activity or delay time. KD qod treated rats (KOD) displayed a slight delay in the place navigation test on P35 compared with the KD group. There were no significant differences in Timm staining among the three groups. In parallel with these changes, KD treatment (both KD and KOD) induced significantly downregulated mRNA levels of Apoa1, Pdk4, and upregulated expression of ApoE, ANXN7, and cPLA2 in the hippocampus when compared with the ND group (except in the case of ApoE in the KOD group). Notably, both the mRNA and protein levels of cPLA2 in the KOD rats were significantly downregulated compared with the KD group but still markedly higher than in the ND group. No significant difference was found in ZnTs among the three groups. Our data suggest that early-life KD can provoke minor neurobehavioral effects in particular a delay in negative geotaxis reflex and an increase in grooming activity. The hippocampal lipid metabolism signaling pathway, especially cPLA2, may be the target of the protective effect of KD on long-term brain injury after developmental seizures.
Keywords: ANXN7; ApoE; Apoa1; Pdk4; cPLA2; ketogenic diet; wild-type rat; zinc transporter.
Figures


Similar articles
-
Neurobehavioral Deficits in a Rat Model of Recurrent Neonatal Seizures Are Prevented by a Ketogenic Diet and Correlate with Hippocampal Zinc/Lipid Transporter Signals.Biol Trace Elem Res. 2015 Oct;167(2):251-8. doi: 10.1007/s12011-015-0285-8. Epub 2015 Mar 17. Biol Trace Elem Res. 2015. PMID: 25778834
-
Ketogenic diet change cPLA2/clusterin and autophagy related gene expression and correlate with cognitive deficits and hippocampal MFs sprouting following neonatal seizures.Epilepsy Res. 2016 Feb;120:13-8. doi: 10.1016/j.eplepsyres.2015.11.021. Epub 2015 Dec 3. Epilepsy Res. 2016. PMID: 26709877
-
Long-Term Effects of Ketogenic Diet on Subsequent Seizure-Induced Brain Injury During Early Adulthood: Relationship of Seizure Thresholds to Zinc Transporter-Related Gene Expressions.Biol Trace Elem Res. 2016 Dec;174(2):369-376. doi: 10.1007/s12011-016-0730-3. Epub 2016 May 5. Biol Trace Elem Res. 2016. PMID: 27147436
-
[Effect of ketogenic diet on hippocampus synaptic reorganization and GluR5 expression in kainic acid induced rat model of epilepsy].Zhonghua Er Ke Za Zhi. 2006 Feb;44(2):100-4. Zhonghua Er Ke Za Zhi. 2006. PMID: 16624024 Chinese.
-
Long-Term Effects of Zinc Deficiency and Zinc Supplementation on Developmental Seizure-Induced Brain Damage and the Underlying GPR39/ZnT-3 and MBP Expression in the Hippocampus.Front Neurosci. 2019 Sep 4;13:920. doi: 10.3389/fnins.2019.00920. eCollection 2019. Front Neurosci. 2019. PMID: 31551684 Free PMC article.
Cited by
-
Potential for Ketotherapies as Amyloid-Regulating Treatment in Individuals at Risk for Alzheimer's Disease.Front Neurosci. 2022 Jun 16;16:899612. doi: 10.3389/fnins.2022.899612. eCollection 2022. Front Neurosci. 2022. PMID: 35784855 Free PMC article. Review.
-
Intrapartum Results on Differing Degrees of Ketonuria in Nulliparous Women with Gestational Diabetes Mellitus during Spontaneous Labor.Int J Endocrinol. 2019 Nov 11;2019:7207012. doi: 10.1155/2019/7207012. eCollection 2019. Int J Endocrinol. 2019. PMID: 31827509 Free PMC article.
-
PRG5 Knockout Precipitates Late-Onset Hypersusceptibility to Pilocarpine-Induced Juvenile Seizures by Exacerbating Hippocampal Zinc Signaling-Mediated Mitochondrial Damage.Front Neurosci. 2021 Aug 27;15:715555. doi: 10.3389/fnins.2021.715555. eCollection 2021. Front Neurosci. 2021. PMID: 34512249 Free PMC article.
-
Ketogenic diet for mood disorders from animal models to clinical application.J Neural Transm (Vienna). 2023 Sep;130(9):1195-1205. doi: 10.1007/s00702-023-02620-x. Epub 2023 Mar 21. J Neural Transm (Vienna). 2023. PMID: 36943505 Free PMC article. Review.
-
The Role of Ketogenic Diet in the Treatment of Neurological Diseases.Nutrients. 2022 Nov 24;14(23):5003. doi: 10.3390/nu14235003. Nutrients. 2022. PMID: 36501033 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

