Dietary Omega-3 Polyunsaturated Fatty Acid Deprivation Does Not Alter Seizure Thresholds but May Prevent the Anti-seizure Effects of Injected Docosahexaenoic Acid in Rats
- PMID: 30804888
- PMCID: PMC6370649
- DOI: 10.3389/fneur.2018.01188
Dietary Omega-3 Polyunsaturated Fatty Acid Deprivation Does Not Alter Seizure Thresholds but May Prevent the Anti-seizure Effects of Injected Docosahexaenoic Acid in Rats
Abstract
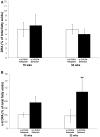
Background: Brain concentrations of omega-3 docosahexaenoic acid (DHA, 22:6n-3) have been reported to positively correlate with seizure thresholds in rodent seizure models. It is not known whether brain DHA depletion, achieved by chronic dietary omega-3 polyunsaturated fatty acid (PUFA) deficiency, lowers seizure thresholds in rats. Objective: The present study tested the hypothesis that lowering brain DHA concentration with chronic dietary n-3 PUFA deprivation in rats will reduce seizure thresholds, and that compared to injected oleic acid (OA), injected DHA will raise seizure thresholds in rats maintained on n-3 PUFA adequate and deficient diets. Methods: Rats (60 days old) were surgically implanted with electrodes in the amygdala, and subsequently randomized to the AIN-93G diet containing adequate levels of n-3 PUFA derived from soybean oil or an n-3 PUFA-deficient diet derived from coconut and safflower oil. The rats were maintained on the diets for 37 weeks. Afterdischarge seizure thresholds (ADTs) were measured every 4-6 weeks by electrically stimulating the amygdala. Between weeks 35 and 37, ADTs were assessed within 1 h of subcutaneous OA or DHA injection (600 mg/kg). Seizure thresholds were also measured in a parallel group of non-implanted rats subjected to the maximal pentylenetetrazol (PTZ, 110 mg/kg) seizure test. PUFA composition was measured in the pyriform-amygdala complex of another group of non-implanted rats sacrificed at 16 and 32 weeks. Results: Dietary n-3 PUFA deprivation did not significantly alter amygdaloid seizure thresholds or latency to PTZ-induced seizures. Acute injection of OA did not alter amygdaloid ADTs of rats on the n-3 PUFA adequate or deficient diets, whereas acute injection of DHA significantly increased amygdaloid ADTs in rats on the n-3 PUFA adequate control diet as compared to rats on the n-3 PUFA deficient diet (P < 0.05). Pyriform-amygdala DHA percent composition did not significantly differ between the groups, while n-6 docosapentaenoic acid, a marker of n-3 PUFA deficiency, was significantly increased by 2.9-fold at 32 weeks. Conclusion: Chronic dietary n-3 PUFA deficiency does not alter seizure thresholds in rats, but may prevent the anti-seizure effects of DHA.
Keywords: DHA; after-discharge seizure threshold; amygdala; omega-3 deficiency; pentylenetetrazol.
Figures





Similar articles
-
A minimum of 3 months of dietary fish oil supplementation is required to raise amygdaloid afterdischarge seizure thresholds in rats--implications for treating complex partial seizures.Epilepsy Behav. 2013 Apr;27(1):49-58. doi: 10.1016/j.yebeh.2012.12.004. Epub 2013 Jan 31. Epilepsy Behav. 2013. PMID: 23376336
-
Acute administration of docosahexaenoic acid increases resistance to pentylenetetrazol-induced seizures in rats.Epilepsy Behav. 2010 Mar;17(3):336-43. doi: 10.1016/j.yebeh.2010.01.001. Epub 2010 Feb 13. Epilepsy Behav. 2010. PMID: 20153982
-
Intraperitoneal administration of docosahexaenoic acid for 14days increases serum unesterified DHA and seizure latency in the maximal pentylenetetrazol model.Epilepsy Behav. 2014 Apr;33:138-43. doi: 10.1016/j.yebeh.2014.02.020. Epub 2014 Mar 22. Epilepsy Behav. 2014. PMID: 24662925
-
Omega-3 fatty acids and neurological injury.Prostaglandins Leukot Essent Fatty Acids. 2007 Nov-Dec;77(5-6):295-300. doi: 10.1016/j.plefa.2007.10.021. Epub 2007 Nov 26. Prostaglandins Leukot Essent Fatty Acids. 2007. PMID: 18036801 Review.
-
Effects of docosahexaenoic Acid on neurotransmission.Biomol Ther (Seoul). 2012 Mar;20(2):152-7. doi: 10.4062/biomolther.2012.20.2.152. Biomol Ther (Seoul). 2012. PMID: 24116288 Free PMC article. Review.
Cited by
-
Dietary oleic acid contributes to the regulation of food intake through the synthesis of intestinal oleoylethanolamide.Front Endocrinol (Lausanne). 2023 Jan 17;13:1056116. doi: 10.3389/fendo.2022.1056116. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36733808 Free PMC article.
References
-
- Burnham WM. Antiseizure drugs. In: Kalant H, Grant DM, Mitchell J. editors. Principles of Medical Pharmacology. Toronto, ON: Saunders Elsevier; (2007). p. 223–35.

