Past, Present, and Future of Regulatory T Cell Therapy in Transplantation and Autoimmunity
- PMID: 30804926
- PMCID: PMC6371029
- DOI: 10.3389/fimmu.2019.00043
Past, Present, and Future of Regulatory T Cell Therapy in Transplantation and Autoimmunity
Abstract
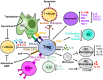
Regulatory T cells (Tregs) are important for the induction and maintenance of peripheral tolerance therefore, they are key in preventing excessive immune responses and autoimmunity. In the last decades, several reports have been focussed on understanding the biology of Tregs and their mechanisms of action. Preclinical studies have demonstrated the ability of Tregs to delay/prevent graft rejection and to control autoimmune responses following adoptive transfer in vivo. Due to these promising results, Tregs have been extensively studied as a potential new tool for the prevention of graft rejection and/or the treatment of autoimmune diseases. Currently, solid organ transplantation remains the treatment of choice for end-stage organ failure. However, chronic rejection and the ensuing side effects of immunosuppressants represent the main limiting factors for organ acceptance and patient survival. Autoimmune disorders are chronic diseases caused by the breakdown of tolerance against self-antigens. This is triggered either by a numerical or functional Treg defect, or by the resistance of effector T cells to suppression. In this scenario, patients receiving high doses of immunosuppressant are left susceptible to life-threatening opportunistic infections and have increased risk of malignancies. In the last 10 years, a few phase I clinical trials aiming to investigate safety and feasibility of Treg-based therapy have been completed and published, whilst an increasing numbers of trials are still ongoing. The first results showed safety and feasibility of Treg therapy and phase II clinical trials are already enrolling. In this review, we describe our understanding of Tregs focussing primarily on their ontogenesis, mechanisms of action and methods used in the clinic for isolation and expansion. Furthermore, we will describe the ongoing studies and the results from the first clinical trials with Tregs in the setting of solid organ transplantation and autoimmune disorders. Finally, we will discuss strategies to further improve the success of Treg therapy.
Keywords: Tregs (regulatory T cells); autoimmunity; cell therapy; clinical trial; transplantation.
Figures
References
-
- Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science (1969) 166:753–5. - PubMed
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. (1995) 155:1151–64. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

