Non-canonical Inflammasome-Mediated IL-1β Production by Primary Endometrial Epithelial and Stromal Fibroblast Cells Is NLRP3 and Caspase-4 Dependent
- PMID: 30804935
- PMCID: PMC6371858
- DOI: 10.3389/fimmu.2019.00102
Non-canonical Inflammasome-Mediated IL-1β Production by Primary Endometrial Epithelial and Stromal Fibroblast Cells Is NLRP3 and Caspase-4 Dependent
Abstract
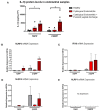
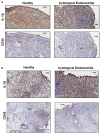
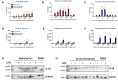
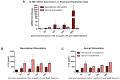
Inflammation of the post-partum uterus is a normal physiological event, crucial for tissue involution and repair. However, in the bovine, some cows fail to resolve this inflammation, resulting in endometritis, which compromises fertility. Earlier work has identified upregulated expression of the potent inflammatory cytokine IL-1β early post-partum, in cows which subsequently develop endometritis. As a result, targeting IL-1β expression holds potential as a novel treatment for this disease, yet the regulatory mechanisms contributing to IL-1β expression in the bovine endometrium remain unknown. To investigate this, endometrial tissue samples were obtained 7 and 21 days post-partum (DPP) from cows that were diagnosed with endometritis at 21 DPP and cows that experienced a physiological level of inflammation throughout involution. IL-1β was measured by qPCR, ELISA, and immunohistochemistry. Seven DPP, endometrial IL-1β protein levels were significantly higher in animals that proceeded to develop endometritis at 21 DPP. IL-1β production could be detected in luminal and glandular epithelium, in underlying stromal fibroblasts as well as infiltrating immune cells. To investigate the mechanisms regulating IL-1β expression, primary endometrial epithelial cells, stromal fibroblasts and PBMCs were stimulated with LPS and the inflammasome activator nigericin. Stromal fibroblast cells were particularly potent producers of IL-1β. Basolateral LPS stimulation of polarized epithelial cells induced IL1B mRNA and a previously undescribed IL-1β protein isoform, with preferential protein secretion into the apical compartment. Key inflammasome components [nod-like receptor protein 3 (NLRP3), nima-related kinase-7 (NEK7), apoptosis speck like protein containing a CARD (ASC), and gasdermin-D] were expressed by endometrial cells following stimulation. Endometrial cell stimulation in the presence of NLRP3 receptor (MCC950) and pan-caspase (Z-VAD-FMK) inhibitors blocked IL-1β production, demonstrating its dependence on the NLRP3 inflammasome and on caspase activity. Furthermore, caspase-4 specific siRNA prevented IL-1β production, confirming that inflammasome activation in endometrial cells is caspase-4 but not caspase-1 dependent, as shown in other species. Identifying the tissue- and species-specificity of inflammasome assembly and activation has critical relevance for our understanding of inflammation and suggests new therapeutic targets to enhance the resolution of inflammatory pathologies including endometritis in cattle.
Keywords: IL-1β; NLRP3 inflammasome; endometritis; epithelial cells; inflammation; stromal fibroblasts.
Figures
Similar articles
-
Endometrial inflammasome activation accompanies menstruation and may have implications for systemic inflammatory events of the menstrual cycle.Hum Reprod. 2020 Jun 1;35(6):1363-1376. doi: 10.1093/humrep/deaa065. Hum Reprod. 2020. PMID: 32488243
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
Protective effect of the total flavonoids from Clinopodium chinense against LPS-induced mice endometritis by inhibiting NLRP3 inflammasome-mediated pyroptosis.J Ethnopharmacol. 2023 Aug 10;312:116489. doi: 10.1016/j.jep.2023.116489. Epub 2023 Apr 11. J Ethnopharmacol. 2023. PMID: 37054825
-
Recent advances in the NEK7-licensed NLRP3 inflammasome activation: Mechanisms, role in diseases and related inhibitors.J Autoimmun. 2020 Sep;113:102515. doi: 10.1016/j.jaut.2020.102515. Epub 2020 Jul 20. J Autoimmun. 2020. PMID: 32703754 Review.
-
Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation.Annu Rev Immunol. 2023 Apr 26;41:301-316. doi: 10.1146/annurev-immunol-081022-021207. Epub 2023 Feb 7. Annu Rev Immunol. 2023. PMID: 36750315 Free PMC article. Review.
Cited by
-
The Crucial Role of NLRP3 Inflammasome in Viral Infection-Associated Fibrosing Interstitial Lung Diseases.Int J Mol Sci. 2021 Sep 28;22(19):10447. doi: 10.3390/ijms221910447. Int J Mol Sci. 2021. PMID: 34638790 Free PMC article. Review.
-
Cytochalasin B Mitigates the Inflammatory Response in Lipopolysaccharide-Induced Mastitis by Suppressing Both the ARPC3/ARPC4-Dependent Cytoskeletal Changes and the Association Between HSP70 and the NLRP3 Inflammasome.Int J Mol Sci. 2025 Mar 26;26(7):3029. doi: 10.3390/ijms26073029. Int J Mol Sci. 2025. PMID: 40243637 Free PMC article.
-
Modulation of Bovine Endometrial Cell Receptors and Signaling Pathways as a Nanotherapeutic Exploration against Dairy Cow Postpartum Endometritis.Animals (Basel). 2021 May 23;11(6):1516. doi: 10.3390/ani11061516. Animals (Basel). 2021. PMID: 34071093 Free PMC article. Review.
-
Acanthamoeba castellanii-Mediated Reduction of Interleukin-1β Secretion and Its Association With Macrophage Autophagy.Scientifica (Cairo). 2025 Mar 12;2025:3430892. doi: 10.1155/sci5/3430892. eCollection 2025. Scientifica (Cairo). 2025. PMID: 40109888 Free PMC article.
-
In Vitro Analysis of LPS-Induced miRNA Differences in Bovine Endometrial Cells and Study of Related Pathways.Animals (Basel). 2024 Nov 22;14(23):3367. doi: 10.3390/ani14233367. Animals (Basel). 2024. PMID: 39682333 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

