HIF-1α-Deficiency in Myeloid Cells Leads to a Disturbed Accumulation of Myeloid Derived Suppressor Cells (MDSC) During Pregnancy and to an Increased Abortion Rate in Mice
- PMID: 30804946
- PMCID: PMC6370686
- DOI: 10.3389/fimmu.2019.00161
HIF-1α-Deficiency in Myeloid Cells Leads to a Disturbed Accumulation of Myeloid Derived Suppressor Cells (MDSC) During Pregnancy and to an Increased Abortion Rate in Mice
Abstract
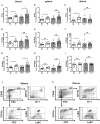
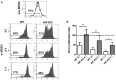
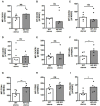
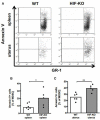
Abortions are the most important reason for unintentional childlessness. During pregnancy, maternal immune cells are in close contact to cells of the semi-allogeneic fetus. Dysregulation of the maternal immune system leading to defective adaptation to pregnancy often plays a role in pathogenesis of abortions. Myeloid-derived suppressor cells (MDSC) are myeloid cells that suppress functions of other immune cells, especially T-cells, thereby negatively affecting diseases such as cancer, sepsis or trauma. They seem, however, also necessary for maintenance of maternal-fetal tolerance. Mechanisms regulating MDSC expansion and function during pregnancy are only incompletely understood. In tumor environment, hypoxia is crucial for MDSC accumulation and activation. Hypoxia is also important for early placenta and embryo development. Effects of hypoxia are mediated through hypoxia-inducible factor 1α (HIF-1α). In the present study we aimed to examine the role of HIF-1α in myeloid cells for MDSC accumulation and MDSC function during pregnancy and for pregnancy outcome. We therefore used a mouse model with targeted deletion of HIF-1α in myeloid cells (myeloid HIF-KO) and analyzed blood, spleens and uteri of pregnant mice at gestational day E 10.5 in comparison to non-pregnant animals and wildtype (WT) animals. Further we analyzed pregnancy success by determining rates of failed implantation and abortion in WT and myeloid HIF-KO animals. We found that myeloid HIF-KO in mice led to an abrogated MDSC accumulation in the pregnant uterus and to impaired suppressive activity of MDSC. While expression of chemokine receptors and integrins on MDSC was not affected by HIF-1α, myeloid HIF-KO led to increased apoptosis rates of MDSC in the uterus. Myeloid-HIF-KO resulted in increased proportions of non-pregnant animals after positive vaginal plug and increased abortion rates, suggesting that activation of HIF-1α dependent pathways in MDSC are important for maintenance of pregnancy.
Keywords: HIF; MDSC; abortion; apoptosis; pregnancy.
Figures

Similar articles
-
HIF-1α targeted deletion in myeloid cells decreases MDSC accumulation and alters microbiome in neonatal mice.Eur J Immunol. 2023 Jul;53(7):e2250144. doi: 10.1002/eji.202250144. Epub 2023 Apr 26. Eur J Immunol. 2023. PMID: 37044112
-
Myeloid-derived suppressor cells are essential for maintaining feto-maternal immunotolerance via STAT3 signaling in mice.J Leukoc Biol. 2016 Sep;100(3):499-511. doi: 10.1189/jlb.1A1015-481RR. Epub 2016 Feb 26. J Leukoc Biol. 2016. PMID: 27203698
-
Lactate accumulation from HIF-1α-mediated PMN-MDSC glycolysis restricts brain injury after acute hypoxia in neonates.J Neuroinflammation. 2025 Mar 1;22(1):59. doi: 10.1186/s12974-025-03385-8. J Neuroinflammation. 2025. PMID: 40025545 Free PMC article.
-
Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment.J Immunol. 2018 Jan 15;200(2):422-431. doi: 10.4049/jimmunol.1701019. J Immunol. 2018. PMID: 29311384 Free PMC article. Review.
-
Hypoxia-inducible factor-1α regulation of myeloid cells.J Mol Med (Berl). 2018 Dec;96(12):1293-1306. doi: 10.1007/s00109-018-1710-1. Epub 2018 Nov 1. J Mol Med (Berl). 2018. PMID: 30386909 Free PMC article. Review.
Cited by
-
Pilot Study on the Effect of Patient Condition and Clinical Parameters on Hypoxia-Induced Factor Expression: HIF1A, EPAS1 and HIF3A in Human Colostrum Cells.Int J Mol Sci. 2024 Oct 14;25(20):11042. doi: 10.3390/ijms252011042. Int J Mol Sci. 2024. PMID: 39456823 Free PMC article.
-
Exploring the Potential of Glycolytic Modulation in Myeloid-Derived Suppressor Cells for Immunotherapy and Disease Management.Immune Netw. 2024 Jun 24;24(3):e26. doi: 10.4110/in.2024.24.e26. eCollection 2024 Jun. Immune Netw. 2024. PMID: 38974210 Free PMC article. Review.
-
Deciphering the preeclampsia-specific immune microenvironment and the role of pro-inflammatory macrophages at the maternal-fetal interface.Elife. 2025 Mar 28;13:RP100002. doi: 10.7554/eLife.100002. Elife. 2025. PMID: 40152904 Free PMC article.
-
Human Leucocyte Antigen G and Murine Qa-2 Are Critical for Myeloid Derived Suppressor Cell Expansion and Activation and for Successful Pregnancy Outcome.Front Immunol. 2022 Jan 17;12:787468. doi: 10.3389/fimmu.2021.787468. eCollection 2021. Front Immunol. 2022. PMID: 35111157 Free PMC article.
-
Identification of chromosomal abnormalities in miscarriages by CNV-Seq.Mol Cytogenet. 2024 Feb 18;17(1):4. doi: 10.1186/s13039-024-00671-7. Mol Cytogenet. 2024. PMID: 38369498 Free PMC article.
References
-
- (AGIM) D.G.f.G.u.G.D.u.d.A.I.i.d.G.u.G (2013). Diagnostik und therapie beim wiederholten Spontanabort. AWMF-Register 015/050.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

