Interference With Quorum-Sensing Signal Biosynthesis as a Promising Therapeutic Strategy Against Multidrug-Resistant Pathogens
- PMID: 30805311
- PMCID: PMC6371041
- DOI: 10.3389/fcimb.2018.00444
Interference With Quorum-Sensing Signal Biosynthesis as a Promising Therapeutic Strategy Against Multidrug-Resistant Pathogens
Abstract
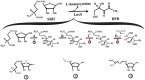
Faced with the global health threat of increasing resistance to antibiotics, researchers are exploring interventions that target bacterial virulence factors. Quorum sensing is a particularly attractive target because several bacterial virulence factors are controlled by this mechanism. Furthermore, attacking the quorum-sensing signaling network is less likely to select for resistant strains than using conventional antibiotics. Strategies that focus on the inhibition of quorum-sensing signal production are especially attractive because the enzymes involved are expressed in bacterial cells but are not present in their mammalian counterparts. We review here various approaches that are being taken to interfere with quorum-sensing signal production via the inhibition of autoinducer-2 synthesis, PQS synthesis, peptide autoinducer synthesis, and N-acyl-homoserine lactone synthesis. We expect these approaches will lead to the discovery of new quorum-sensing inhibitors that can help to stem the tide of antibiotic resistance.
Keywords: anti-virulence therapy; antibiotic resistance; quorum sensing; quorum-sensing inhibition; virulence.
Figures

Similar articles
-
Itaconimides as Novel Quorum Sensing Inhibitors of Pseudomonas aeruginosa.Front Cell Infect Microbiol. 2019 Jan 7;8:443. doi: 10.3389/fcimb.2018.00443. eCollection 2018. Front Cell Infect Microbiol. 2019. PMID: 30666301 Free PMC article.
-
Recent Advances in Anti-virulence Therapeutic Strategies With a Focus on Dismantling Bacterial Membrane Microdomains, Toxin Neutralization, Quorum-Sensing Interference and Biofilm Inhibition.Front Cell Infect Microbiol. 2019 Apr 2;9:74. doi: 10.3389/fcimb.2019.00074. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31001485 Free PMC article. Review.
-
Recent Advances in Ligand and Structure Based Screening of Potent Quorum Sensing Inhibitors Against Antibiotic Resistance Induced Bacterial Virulence.Recent Pat Biotechnol. 2016;10(2):195-216. doi: 10.2174/1872208310666160728104450. Recent Pat Biotechnol. 2016. PMID: 27468815 Review.
-
Quorum-Sensing Systems as Targets for Antivirulence Therapy.Trends Microbiol. 2018 Apr;26(4):313-328. doi: 10.1016/j.tim.2017.10.005. Epub 2017 Nov 10. Trends Microbiol. 2018. PMID: 29132819 Review.
-
Bacterial quorum sensing inhibitors: attractive alternatives for control of infectious pathogens showing multiple drug resistance.Recent Pat Antiinfect Drug Discov. 2013 Apr;8(1):68-83. doi: 10.2174/1574891x11308010012. Recent Pat Antiinfect Drug Discov. 2013. PMID: 23394143 Review.
Cited by
-
Phomoxanthone A, Compound of Endophytic Fungi Paecilomyces sp. and Its Potential Antimicrobial and Antiparasitic.Antibiotics (Basel). 2022 Sep 29;11(10):1332. doi: 10.3390/antibiotics11101332. Antibiotics (Basel). 2022. PMID: 36289990 Free PMC article.
-
How does Quorum Sensing of Intestinal Bacteria Affect Our Health and Mental Status?Microorganisms. 2022 Oct 5;10(10):1969. doi: 10.3390/microorganisms10101969. Microorganisms. 2022. PMID: 36296244 Free PMC article. Review.
-
Metabolomic Analysis of the Effect of Lippia origanoides Essential Oil on the Inhibition of Quorum Sensing in Chromobacterium violaceum.Antibiotics (Basel). 2023 Apr 26;12(5):814. doi: 10.3390/antibiotics12050814. Antibiotics (Basel). 2023. PMID: 37237719 Free PMC article.
-
The role of bacterial signaling networks in antibiotics response and resistance regulation.Mar Life Sci Technol. 2022 Mar 28;4(2):163-178. doi: 10.1007/s42995-022-00126-1. eCollection 2022 May. Mar Life Sci Technol. 2022. PMID: 37073223 Free PMC article. Review.
-
Pseudomonas aeruginosa biofilm: treatment strategies to combat infection.Arch Microbiol. 2025 May 10;207(6):141. doi: 10.1007/s00203-025-04346-8. Arch Microbiol. 2025. PMID: 40348909 Review.
References
-
- Armbruster C. E., Hong W., Pang B., Dew K.E., Juneau R. A., Byrd M. S., et al. (2009). LuxS promote biofilms maturation and persistence of nontypeable Haemophilus influenzae in vivo via modulation of lipooligosccharides on bacterial surface. Infect. Immun. 77, 4081–4019. 10.1128/IAI.00320-09 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

