Ossified blood vessels in primary familial brain calcification elicit a neurotoxic astrocyte response
- PMID: 30805583
- PMCID: PMC6439320
- DOI: 10.1093/brain/awz032
Ossified blood vessels in primary familial brain calcification elicit a neurotoxic astrocyte response
Abstract
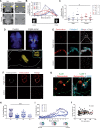
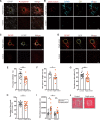
Brain calcifications are commonly detected in aged individuals and accompany numerous brain diseases, but their functional importance is not understood. In cases of primary familial brain calcification, an autosomally inherited neuropsychiatric disorder, the presence of bilateral brain calcifications in the absence of secondary causes of brain calcification is a diagnostic criterion. To date, mutations in five genes including solute carrier 20 member 2 (SLC20A2), xenotropic and polytropic retrovirus receptor 1 (XPR1), myogenesis regulating glycosidase (MYORG), platelet-derived growth factor B (PDGFB) and platelet-derived growth factor receptor β (PDGFRB), are considered causal. Previously, we have reported that mutations in PDGFB in humans are associated with primary familial brain calcification, and mice hypomorphic for PDGFB (Pdgfbret/ret) present with brain vessel calcifications in the deep regions of the brain that increase with age, mimicking the pathology observed in human mutation carriers. In this study, we characterize the cellular environment surrounding calcifications in Pdgfbret/ret animals and show that cells around vessel-associated calcifications express markers for osteoblasts, osteoclasts and osteocytes, and that bone matrix proteins are present in vessel-associated calcifications. Additionally, we also demonstrate the osteogenic environment around brain calcifications in genetically confirmed primary familial brain calcification cases. We show that calcifications cause oxidative stress in astrocytes and evoke expression of neurotoxic astrocyte markers. Similar to previously reported human primary familial brain calcification cases, we describe high interindividual variation in calcification load in Pdgfbret/ret animals, as assessed by ex vivo and in vivo quantification of calcifications. We also report that serum of Pdgfbret/ret animals does not differ in calcification propensity from control animals and that vessel calcification occurs only in the brains of Pdgfbret/ret animals. Notably, ossification of vessels and astrocytic neurotoxic response is associated with specific behavioural and cognitive alterations, some of which are associated with primary familial brain calcification in a subset of patients.
Keywords: PDGFB; neurotoxic astrocyte; ossification; prepulse inhibition; primary familial brain calcification.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



Comment in
-
Reply: Osteoclast imbalance in primary familial brain calcification: evidence for its role in brain calcification.Brain. 2020 Jan 1;143(1):e2. doi: 10.1093/brain/awz352. Brain. 2020. PMID: 31754684 No abstract available.
-
Osteoclast imbalance in primary familial brain calcification: evidence for its role in brain calcification.Brain. 2020 Jan 1;143(1):e1. doi: 10.1093/brain/awz351. Brain. 2020. PMID: 31754706 No abstract available.
References
-
- Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 2007; 116: 2841–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

