Interictal and ictal source localization for epilepsy surgery using high-density EEG with MEG: a prospective long-term study
- PMID: 30805596
- PMCID: PMC6459284
- DOI: 10.1093/brain/awz015
Interictal and ictal source localization for epilepsy surgery using high-density EEG with MEG: a prospective long-term study
Abstract
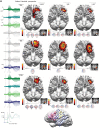
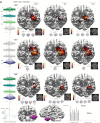
Drug-resistant focal epilepsy is a major clinical problem and surgery is under-used. Better non-invasive techniques for epileptogenic zone localization are needed when MRI shows no lesion or an extensive lesion. The problem is interictal and ictal localization before propagation from the epileptogenic zone. High-density EEG (HDEEG) and magnetoencephalography (MEG) offer millisecond-order temporal resolution to address this but co-acquisition is challenging, ictal MEG studies are rare, long-term prospective studies are lacking, and fundamental questions remain. Should HDEEG-MEG discharges be assessed independently [electroencephalographic source localization (ESL), magnetoencephalographic source localization (MSL)] or combined (EMSL) for source localization? Which phase of the discharge best characterizes the epileptogenic zone (defined by intracranial EEG and surgical resection relative to outcome)? Does this differ for interictal and ictal discharges? Does MEG detect mesial temporal lobe discharges? Thirteen patients (10 non-lesional, three extensive-lesional) underwent synchronized HDEEG-MEG (72-94 channel EEG, 306-sensor MEG). Source localization (standardized low-resolution tomographic analysis with MRI patient-individualized boundary-element method) was applied to averaged interictal epileptiform discharges (IED) and ictal discharges at three phases: 'early-phase' (first latency 90% explained variance), 'mid-phase' (first of 50% rising-phase, 50% mean global field power), 'late-phase' (negative peak). 'Earliest-solution' was the first of the three early-phase solutions (ESL, MSL, EMSL). Prospective follow-up was 3-21 (median 12) months before surgery, 14-39 (median 21) months after surgery. IEDs (n = 1474) were recorded, seen in: HDEEG only, 626 (42%); MEG only, 232 (16%); and both 616 (42%). Thirty-three seizures were captured, seen in: HDEEG only, seven (21%); MEG only, one (3%); and both 25 (76%). Intracranial EEG was done in nine patients. Engel scores were I (9/13, 69%), II (2/13,15%), and III (2/13). MEG detected baso-mesial temporal lobe epileptogenic zone sources. Epileptogenic zone OR [odds ratio(s)] were significantly higher for earliest-solution versus early-phase IED-surgical resection and earliest-solution versus all mid-phase and late-phase solutions. ESL outperformed EMSL for ictal-surgical resection [OR 3.54, 95% confidence interval (CI) 1.09-11.55, P = 0.036]. MSL outperformed EMSL for IED-intracranial EEG (OR 4.67, 95% CI 1.19-18.34, P = 0.027). ESL outperformed MSL for ictal-surgical resection (OR 3.73, 95% CI 1.16-12.03, P = 0.028) but was outperformed by MSL for IED-intracranial EEG (OR 0.18, 95% CI 0.05-0.73, P = 0.017). Thus, (i) HDEEG and MEG source solutions more accurately localize the epileptogenic zone at the earliest resolvable phase of interictal and ictal discharges, not mid-phase (as is common practice) or late peak-phase (when signal-to-noise ratios are maximal); (ii) from empirical observation of the differential timing of HDEEG and MEG discharges and based on the superiority of ESL plus MSL over either modality alone and over EMSL, concurrent HDEEG-MEG signals should be assessed independently, not combined; (iii) baso-mesial temporal lobe sources are detectable by MEG; and (iv) MEG is not 'more accurate' than HDEEG-emphasis is best placed on the earliest signal (whether HDEEG or MEG) amenable to source localization. Our findings challenge current practice and our reliance on invasive monitoring in these patients. 10.1093/brain/awz015_video1 awz015media1 6018582479001.
Keywords: combined electromagnetoencephalographic source localization; electroencephalographic source localization; high density electroencephalography; magnetoencephalographic source localization; magnetoencephalography.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures




Similar articles
-
Accuracy of MEG in localizing irritative zone and seizure onset zone: Quantitative comparison between MEG and intracranial EEG.Epilepsy Res. 2016 Nov;127:291-301. doi: 10.1016/j.eplepsyres.2016.08.013. Epub 2016 Aug 16. Epilepsy Res. 2016. PMID: 27693985
-
Multimodal and quantitative analysis of the epileptogenic zone network in the pre-surgical evaluation of drug-resistant focal epilepsy.Neurophysiol Clin. 2024 Nov;54(6):103021. doi: 10.1016/j.neucli.2024.103021. Epub 2024 Oct 25. Neurophysiol Clin. 2024. PMID: 39461243
-
Source localization determined by magnetoencephalography and electroencephalography in temporal lobe epilepsy: comparison with electrocorticography: technical case report.Neurosurgery. 1998 Feb;42(2):414-21; discussion 421-2. doi: 10.1097/00006123-199802000-00142. Neurosurgery. 1998. PMID: 9482198
-
Electroencephalography, magnetoencephalography and source localization: their value in epilepsy.Curr Opin Neurol. 2018 Apr;31(2):176-183. doi: 10.1097/WCO.0000000000000545. Curr Opin Neurol. 2018. PMID: 29432218 Review.
-
MEG and EEG in epilepsy.J Clin Neurophysiol. 2003 May-Jun;20(3):163-78. doi: 10.1097/00004691-200305000-00002. J Clin Neurophysiol. 2003. PMID: 12881663 Review.
Cited by
-
Source imaging of seizure onset predicts surgical outcome in pediatric epilepsy.Clin Neurophysiol. 2021 Jul;132(7):1622-1635. doi: 10.1016/j.clinph.2021.03.043. Epub 2021 Apr 28. Clin Neurophysiol. 2021. PMID: 34034087 Free PMC article.
-
Localizing the Epileptogenic Zone with Novel Biomarkers.Semin Pediatr Neurol. 2021 Oct;39:100919. doi: 10.1016/j.spen.2021.100919. Epub 2021 Aug 20. Semin Pediatr Neurol. 2021. PMID: 34620466 Free PMC article. Review.
-
Surgical cure of intractable epilepsy caused by retained intracranial foreign body under cortical electroencephalography monitoring: case report and literature review.Front Surg. 2025 Jun 6;12:1614564. doi: 10.3389/fsurg.2025.1614564. eCollection 2025. Front Surg. 2025. PMID: 40546895 Free PMC article.
-
Influences of electrode density on intracranial seizure localisation: a single-blinded randomised crossover study.EBioMedicine. 2025 Mar;113:105606. doi: 10.1016/j.ebiom.2025.105606. Epub 2025 Mar 3. EBioMedicine. 2025. PMID: 40037091 Free PMC article. Clinical Trial.
-
Case Report: Laser Ablation Guided by State of the Art Source Imaging Ends an Adolescent's 16-Year Quest for Seizure Freedom.Front Hum Neurosci. 2022 Jan 25;16:826139. doi: 10.3389/fnhum.2022.826139. eCollection 2022. Front Hum Neurosci. 2022. PMID: 35145387 Free PMC article.
References
-
- Agirre-Arrizubieta Z, Huiskamp GJM, Ferrier CH, van Huffelen AC, Leijten FSS. Interictal magnetoencephalography and the irritative zone in the electrocorticogram. Brain 2009; 132: 3060–71. - PubMed
-
- Baumgartner C, Pataraia E, Lindinger G, Deecke L. Neuromagnetic recordings in temporal lobe epilepsy. J Clin Neurophysiol 2000; 17: 177–89. - PubMed
-
- Choi JH, Jung YJ, Jung HK, Im CH. A new multimodal cortical source imaging algorithm for integrating simultaneously recorded EEG and MEG. Inverse Probl Sci Eng 2013; 21: 1074–89.

