Methadone dosing strategies in preterm neonates can be simplified
- PMID: 30805946
- PMCID: PMC6533437
- DOI: 10.1111/bcp.13906
Methadone dosing strategies in preterm neonates can be simplified
Abstract
Aims: A dramatic increase in newborn infants with neonatal abstinence syndrome has been observed and these neonates are frequently treated with complex methadone dosing schemes to control their withdrawal symptoms. Despite its abundant use, hardly any data on the pharmacokinetics (PK) of methadone is available in preterm neonates. Therefore we investigated developmental PK of methadone and evaluated current dosing strategies and possible simplification in this vulnerable population.
Methods: A single-centre open-label prospective study was performed to collect PK data after a single oral dose of methadone in preterm neonates. A population PK model was built to characterize developmental PK of (R)- and (S)-methadone. Model-based simulations were performed to identify a simplified dosing strategy to reach and maintain target methadone exposure.
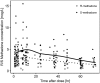
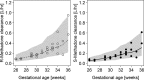
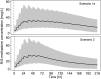
Results: A total of 121 methadone concentrations were collected from 31 preterm neonates. A one-compartment model with first order absorption and elimination kinetics best described PK data for (R)- and (S)-methadone. Clearance increases with advancing gestational age and differs between R- and S-enantiomer, being slightly higher for the former (0.244 vs 0.167 L/h). Preterm neonates reached target exposure after 48 hours with currently used dosing schedules. Output from simulations revealed that target exposures can be achieved with a simplified dosing strategy during the first 4 days of treatment.
Conclusion: Methadone clearance in preterm neonates increases with advancing gestational age and its disposition is influenced by its chirality. Simulations that account for developmental PK changes indicate a shorter methadone dosing strategy can maintain target exposure to control withdrawal symptoms.
Trial registration: ClinicalTrials.gov NCT01327079.
Keywords: dosing optimization; gestational age; methadone; neonatal abstinence syndrome; preterm neonates.
© 2019 The British Pharmacological Society.
Conflict of interest statement
M.P. has part‐time employment with the consulting company Certara, USA.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

