Veins and Arteries Build Hierarchical Branching Patterns Differently: Bottom-Up versus Top-Down
- PMID: 30805984
- PMCID: PMC6478158
- DOI: 10.1002/bies.201800198
Veins and Arteries Build Hierarchical Branching Patterns Differently: Bottom-Up versus Top-Down
Abstract
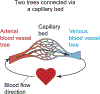
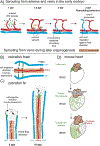
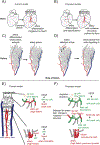
A tree-like hierarchical branching structure is present in many biological systems, such as the kidney, lung, mammary gland, and blood vessels. Most of these organs form through branching morphogenesis, where outward growth results in smaller and smaller branches. However, the blood vasculature is unique in that it exists as two trees (arterial and venous) connected at their tips. Obtaining this organization might therefore require unique developmental mechanisms. As reviewed here, recent data indicate that arterial trees often form in reverse order. Accordingly, initial arterial endothelial cell differentiation occurs outside of arterial vessels. These pre-artery cells then build trees by following a migratory path from smaller into larger arteries, a process guided by the forces imparted by blood flow. Thus, in comparison to other branched organs, arteries can obtain their structure through inward growth and coalescence. Here, new information on the underlying mechanisms is discussed, and how defects can lead to pathologies, such as hypoplastic arteries and arteriovenous malformations.
Keywords: angiogenesis; arteries; blood flow; branching morphogenesis; coronary vasculature; cxcr4; notch; vein.
© 2019 WILEY Periodicals, Inc.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

