Long noncoding RNA DLX6-AS1 promotes liver cancer by increasing the expression of WEE1 via targeting miR-424-5p
- PMID: 30805988
- PMCID: PMC6712946
- DOI: 10.1002/jcb.28493
Long noncoding RNA DLX6-AS1 promotes liver cancer by increasing the expression of WEE1 via targeting miR-424-5p
Abstract
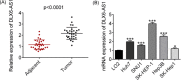
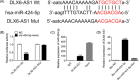
Long noncoding RNAs (lncRNAs) played an important role in tumorigenesis and development of hepatocellular carcinoma (HCC). In this study, we first demonstrated that lncRNA DLX6 antisense RNA 1 (DLX6-AS1) was upregulated in cancer tissues and cells lines compared with normal adjacent and cell line. Knock-down DLX6-AS1 by transfection with small interfering RNA (siRNA) suppressed cell proliferation, migration, and invasion of HCC cells. Cell cycle analysis showed that cells transfected with siRNA were arrested in G0/G1 phase. Then, we performed dual-luciferase reporter assay and RNA immunoprecipitation (RIP) assay to show that DLX6-AS1 could bind with miR-424-5p. And cotransfection inhibitor of miR-424-5p with siRNA of DLX6-AS1 could abolish the inhibitory effect of siRNA of DLX6-AS1 on cell proliferation, migration, and invasion. Moreover, we further demonstrated that the oncogene WEE1 G2 checkpoint kinase (WEE1) was the target of miR-424-5p and expression levels of WEE1 were positive correlation with that of DLX6-AS1. Taken together, these results suggested that upregulated DLX6-AS1 promoted cell proliferation, migration, and invasion of HCC through increasing expression of WEE1 via targeting miR-424-5p.
Keywords: DLX6-AS1; WEE1; hepatocellular carcinoma; long noncoding RNA; miR-424-5p.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



Similar articles
-
DLX6-AS1/miR-204-5p/OCT1 positive feedback loop promotes tumor progression and epithelial-mesenchymal transition in gastric cancer.Gastric Cancer. 2020 Mar;23(2):212-227. doi: 10.1007/s10120-019-01002-1. Epub 2019 Aug 28. Gastric Cancer. 2020. PMID: 31463827
-
Long noncoding RNA UPK1A-AS1 indicates poor prognosis of hepatocellular carcinoma and promotes cell proliferation through interaction with EZH2.J Exp Clin Cancer Res. 2020 Oct 29;39(1):229. doi: 10.1186/s13046-020-01748-y. J Exp Clin Cancer Res. 2020. PMID: 33121524 Free PMC article.
-
Upregulated lncRNA DLX6-AS1 underpins hepatocellular carcinoma progression via the miR-513c/Cul4A/ANXA10 axis.Cancer Gene Ther. 2021 May;28(5):486-501. doi: 10.1038/s41417-020-00233-0. Epub 2020 Dec 4. Cancer Gene Ther. 2021. PMID: 33277615
-
Upregulation of lncRNA NIFK-AS1 in hepatocellular carcinoma by m6A methylation promotes disease progression and sorafenib resistance.Hum Cell. 2021 Nov;34(6):1800-1811. doi: 10.1007/s13577-021-00587-z. Epub 2021 Aug 10. Hum Cell. 2021. PMID: 34374933 Review.
-
DLX6-AS1 is a potential biomarker and therapeutic target in cancer initiation and progression.Clin Chim Acta. 2021 Jun;517:1-8. doi: 10.1016/j.cca.2021.02.006. Epub 2021 Feb 17. Clin Chim Acta. 2021. PMID: 33607068 Review.
Cited by
-
Noncoding RNA-mediated macrophage and cancer cell crosstalk in hepatocellular carcinoma.Mol Ther Oncolytics. 2022 Mar 16;25:98-120. doi: 10.1016/j.omto.2022.03.002. eCollection 2022 Jun 16. Mol Ther Oncolytics. 2022. PMID: 35506150 Free PMC article. Review.
-
Role of miRNA-424 in Cancers.Onco Targets Ther. 2020 Sep 28;13:9611-9622. doi: 10.2147/OTT.S266541. eCollection 2020. Onco Targets Ther. 2020. PMID: 33061443 Free PMC article. Review.
-
DLX6-AS1 activated by H3K4me1 enhanced secondary cisplatin resistance of lung squamous cell carcinoma through modulating miR-181a-5p/miR-382-5p/CELF1 axis.Sci Rep. 2021 Oct 25;11(1):21014. doi: 10.1038/s41598-021-99555-8. Sci Rep. 2021. PMID: 34697393 Free PMC article.
-
Strategies of LncRNA DLX6-AS1 on Study and Therapeutics.Front Genet. 2022 Jun 1;13:871988. doi: 10.3389/fgene.2022.871988. eCollection 2022. Front Genet. 2022. PMID: 35719380 Free PMC article. Review.
-
Promising long noncoding RNA DLX6-AS1 in malignant tumors.Am J Transl Res. 2020 Dec 15;12(12):7682-7692. eCollection 2020. Am J Transl Res. 2020. PMID: 33437353 Free PMC article. Review.
References
-
- Yang JX, Rastetter RH, Wilhelm D. Non‐coding RNAs: an introduction. Adv Exp Med Biol. 2016;886:13‐32. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

