Impaired voice processing in reward and salience circuits predicts social communication in children with autism
- PMID: 30806350
- PMCID: PMC6391069
- DOI: 10.7554/eLife.39906
Impaired voice processing in reward and salience circuits predicts social communication in children with autism
Abstract
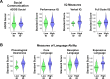
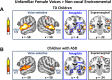
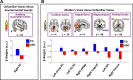
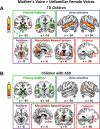
Engaging with vocal sounds is critical for children's social-emotional learning, and children with autism spectrum disorder (ASD) often 'tune out' voices in their environment. Little is known regarding the neurobiological basis of voice processing and its link to social impairments in ASD. Here, we perform the first comprehensive brain network analysis of voice processing in children with ASD. We examined neural responses elicited by unfamiliar voices and mother's voice, a biologically salient voice for social learning, and identified a striking relationship between social communication abilities in children with ASD and activation in key structures of reward and salience processing regions. Functional connectivity between voice-selective and reward regions during voice processing predicted social communication in children with ASD and distinguished them from typically developing children. Results support the Social Motivation Theory of ASD by showing reward system deficits associated with the processing of a critical social stimulus, mother's voice, in children with ASD.
Editorial note: This article has been through an editorial process in which the authors decide how to respond to the issues raised during peer review. The Reviewing Editor's assessment is that minor issues remain unresolved (see decision letter).
Keywords: auditory; autism; brain; human; mother; neuroscience; reward; voice.
© 2019, Abrams et al.
Conflict of interest statement
DA, AP, TC, PO, AB, JK, JP, VM No competing interests declared
Figures







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

