The renin-angiotensin-aldosterone system and its suppression
- PMID: 30806496
- PMCID: PMC6430926
- DOI: 10.1111/jvim.15454
The renin-angiotensin-aldosterone system and its suppression
Erratum in
-
Erratum for The renin-angiotensin-aldosterone system and its suppression.J Vet Intern Med. 2019 Sep;33(5):2551. doi: 10.1111/jvim.15615. Epub 2019 Aug 30. J Vet Intern Med. 2019. PMID: 31565833 Free PMC article. No abstract available.
Abstract
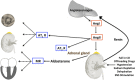
Chronic activation of the renin-angiotensin-aldosterone system (RAAS) promotes and perpetuates the syndromes of congestive heart failure, systemic hypertension, and chronic kidney disease. Excessive circulating and tissue angiotensin II (AngII) and aldosterone levels lead to a pro-fibrotic, -inflammatory, and -hypertrophic milieu that causes remodeling and dysfunction in cardiovascular and renal tissues. Understanding of the role of the RAAS in this abnormal pathologic remodeling has grown over the past few decades and numerous medical therapies aimed at suppressing the RAAS have been developed. Despite this, morbidity from these diseases remains high. Continued investigation into the complexities of the RAAS should help clinicians modulate (suppress or enhance) components of this system and improve quality of life and survival. This review focuses on updates in our understanding of the RAAS and the pathophysiology of AngII and aldosterone excess, reviewing what is known about its suppression in cardiovascular and renal diseases, especially in the cat and dog.
Keywords: angiotensin converting enzyme inhibitor; angiotensin receptor blocker; chronic kidney disease; heart failure; mineralocorticoid receptor blocker; proteinuric kidney disease; systemic hypertension.
© 2019 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals, Inc. on behalf of the American College of Veterinary Internal Medicine.
Conflict of interest statement
Dr Pitt is a consultant for Bayer, Astra Zeneca, Sanofi, Sarfez, scPharmaceuticals, Relypsa/Vifor, Stealth Peptides, Cytopherx (stock options); Dr Atkins is a consultant for Ceva Sante Animale, Vetoquinol, and Boehringer Ingelheim; Dr Ames is a consultant for Ceva Sante Animale, and Elanco.
Figures




References
-
- Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation. 1990;82(5):1730‐1736. - PubMed
-
- Anker SD, Chua TP, Ponikowski P, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96(2):526‐534. - PubMed
-
- Broqvist M, Dahlstrom U, Karlberg BE, et al. Neuroendocrine response in acute heart failure and the influence of treatment. Eur Heart J. 1989;10(12):1075‐1083. - PubMed
-
- Levine TB, Francis GS, Goldsmith SR, Simon AB, Cohn JN. Activity of the sympathetic nervous system and renin‐angiotensin system assessed by plasma hormone levels and their relation to hemodynamic abnormalities in congestive heart failure. Am J Cardiol. 1982;49:1659‐1666. - PubMed
-
- Francis GS, Benedict C, Johnstone DE, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. Circulation. 1990;82:1724‐1729. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
