Association of Genetic Variants in NUDT15 With Thiopurine-Induced Myelosuppression in Patients With Inflammatory Bowel Disease
- PMID: 30806694
- PMCID: PMC6439872
- DOI: 10.1001/jama.2019.0709
Association of Genetic Variants in NUDT15 With Thiopurine-Induced Myelosuppression in Patients With Inflammatory Bowel Disease
Erratum in
-
Numerical Errors and Addition of a Sentence.JAMA. 2019 Apr 23;321(16):1636. doi: 10.1001/jama.2019.3497. JAMA. 2019. PMID: 31012907 Free PMC article. No abstract available.
Abstract
Importance: Use of thiopurines may be limited by myelosuppression. TPMT pharmacogenetic testing identifies only 25% of at-risk patients of European ancestry. Among patients of East Asian ancestry, NUDT15 variants are associated with thiopurine-induced myelosuppression (TIM).
Objective: To identify genetic variants associated with TIM among patients of European ancestry with inflammatory bowel disease (IBD).
Design, setting, and participants: Case-control study of 491 patients affected by TIM and 679 thiopurine-tolerant unaffected patients who were recruited from 89 international sites between March 2012 and November 2015. Genome-wide association studies (GWAS) and exome-wide association studies (EWAS) were conducted in patients of European ancestry. The replication cohort comprised 73 patients affected by TIM and 840 thiopurine-tolerant unaffected patients.
Exposures: Genetic variants associated with TIM.
Main outcomes and measures: Thiopurine-induced myelosuppression, defined as a decline in absolute white blood cell count to 2.5 × 109/L or less or a decline in absolute neutrophil cell count to 1.0 × 109/L or less leading to a dose reduction or drug withdrawal.
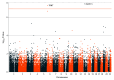
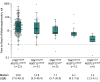
Results: Among 1077 patients (398 affected and 679 unaffected; median age at IBD diagnosis, 31.0 years [interquartile range, 21.2 to 44.1 years]; 540 [50%] women; 602 [56%] diagnosed as having Crohn disease), 919 (311 affected and 608 unaffected) were included in the GWAS analysis and 961 (328 affected and 633 unaffected) in the EWAS analysis. The GWAS analysis confirmed association of TPMT (chromosome 6, rs11969064) with TIM (30.5% [95/311] affected vs 16.4% [100/608] unaffected patients; odds ratio [OR], 2.3 [95% CI, 1.7 to 3.1], P = 5.2 × 10-9). The EWAS analysis demonstrated an association with an in-frame deletion in NUDT15 (chromosome 13, rs746071566) and TIM (5.8% [19/328] affected vs 0.2% [1/633] unaffected patients; OR, 38.2 [95% CI, 5.1 to 286.1], P = 1.3 × 10-8), which was replicated in a different cohort (2.7% [2/73] affected vs 0.2% [2/840] unaffected patients; OR, 11.8 [95% CI, 1.6 to 85.0], P = .03). Carriage of any of 3 coding NUDT15 variants was associated with an increased risk (OR, 27.3 [95% CI, 9.3 to 116.7], P = 1.1 × 10-7) of TIM, independent of TPMT genotype and thiopurine dose.
Conclusions and relevance: Among patients of European ancestry with IBD, variants in NUDT15 were associated with increased risk of TIM. These findings suggest that NUDT15 genotyping may be considered prior to initiation of thiopurine therapy; however, further study including additional validation in independent cohorts is required.
Conflict of interest statement
Figures
Comment in
-
NUDT15 genotyping in Caucasian patients can help to optimise thiopurine treatment in patients with inflammatory bowel disease.Transl Gastroenterol Hepatol. 2019 Dec 12;4:81. doi: 10.21037/tgh.2019.11.09. eCollection 2019. Transl Gastroenterol Hepatol. 2019. PMID: 32039286 Free PMC article. No abstract available.
References
-
- US Food and Drug Administration . Approved drug products with therapeutic equivalence evaluations (Orange Book). https://www.fda.gov/Drugs/InformationOnDrugs/ucm129662.htm. Accessed March 1, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

