Iron uptake by ZIP8 and ZIP14 in human proximal tubular epithelial cells
- PMID: 30806852
- PMCID: PMC6437295
- DOI: 10.1007/s10534-019-00183-7
Iron uptake by ZIP8 and ZIP14 in human proximal tubular epithelial cells
Abstract
In patients with iron overload disorders, increasing number of reports of renal dysfunction and renal iron deposition support an association between increased iron exposure and renal injury. In systemic iron overload, elevated circulating levels of transferrin-bound (TBI) and non-transferrin-bound iron (NTBI) are filtered to the renal proximal tubules, where they may cause injury. However, the mechanisms of tubular iron handling remain elusive. To unravel molecular renal proximal tubular NTBI and TBI handling, human conditionally immortalized proximal tubular epithelial cells (ciPTECs) were incubated with 55Fe as NTBI and fluorescently labeled holo-transferrin as TBI. Ferrous iron importers ZIP8 and ZIP14 were localized in the ciPTEC plasma membrane. Whereas silencing of either ZIP8 or ZIP14 alone did not affect 55Fe uptake, combined silencing significantly reduced 55Fe uptake compared to control (p < 0.05). Furthermore, transferrin receptor 1 (TfR1) and ZIP14, but not ZIP8, colocalized with early endosome antigen 1 (EEA1). TfR1 and ZIP14 also colocalized with uptake of fluorescently labeled transferrin. Furthermore, ZIP14 silencing decreased 55Fe uptake after 55Fe-Transferrin exposure (p < 0.05), suggesting ZIP14 could be involved in early endosomal transport of TBI-derived iron into the cytosol. Our data suggest that human proximal tubular epithelial cells take up TBI and NTBI, where ZIP8 and ZIP14 are both involved in NTBI uptake, but ZIP14, not ZIP8, mediates TBI-derived iron uptake. This knowledge provides more insights in the mechanisms of renal iron handling and suggests that ZIP8 and ZIP14 could be potential targets for limiting renal iron reabsorption and enhancing urinary iron excretion in systemic iron overload disorders.
Keywords: Iron; Non-transferrin-bound iron; Proximal tubular epithelial cell; Transferrin; ZIP14; ZIP8.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures


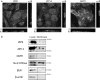

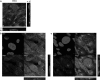

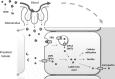
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

