Cyclin E1 Expression and Palbociclib Efficacy in Previously Treated Hormone Receptor-Positive Metastatic Breast Cancer
- PMID: 30807234
- PMCID: PMC6506420
- DOI: 10.1200/JCO.18.00925
Cyclin E1 Expression and Palbociclib Efficacy in Previously Treated Hormone Receptor-Positive Metastatic Breast Cancer
Erratum in
-
Erratum.J Clin Oncol. 2019 Nov 1;37(31):2956. doi: 10.1200/JCO.19.02416. J Clin Oncol. 2019. PMID: 31661657 Free PMC article. No abstract available.
Abstract
Purpose: A large-panel gene expression analysis was conducted to identify biomarkers associated with the effectiveness of adding palbociclib to fulvestrant.
Methods: The PALOMA-3 ( ClinicalTrials.gov identifier: NCT01942135) trial randomly assigned 521 endocrine-pretreated patients with metastatic breast cancer to receive palbociclib plus fulvestrant or placebo plus fulvestrant. Primary analysis was first conducted on 10 genes on the basis of pathway biology and evidence from previous studies followed by a systematic panel-wide search among 2,534 cancer-related genes. The association of gene expression with the effect of palbociclib on progression-free survival (PFS) was evaluated using Cox proportional hazards regression analysis, with gene expression as a continuous variable or dichotomized by median. An independent breast cancer cohort from the Preoperative Palbociclib (POP) Clinical Trial ( ClinicalTrials.gov identifier: NCT02008734) was used for validation, in 61 patients with primary breast cancer treated with 2 weeks of palbociclib.
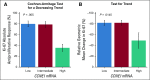
Results: In the PALOMA-3 trial, 302 patients had tumor tissue analyzed (palbociclib arm, 194 patients; placebo arm, 108 patients). Palbociclib efficacy was lower in patients with high versus low cyclin E1 (CCNE1) mRNA expression (median PFS: palbociclib arm, 7.6 v 14.1 months; placebo arm, 4.0 v 4.8 months, respectively; interaction P unadjusted = .00238; false discovery rate-adjusted P = .0238). CCNE1 mRNA was more predictive in metastatic than in archival primary biopsy tissue samples. No significant interaction was found between treatment and expression levels of CDK4, CDK6, cyclin D1, and RB1. Palbociclib was efficacious in both luminal A and luminal B tumors. High CCNE1 mRNA expression was associated with poor antiproliferative activity of palbociclib in the POP trial (P = .005).
Conclusion: Addition of palbociclib to fulvestrant demonstrated efficacy in all biomarker groups, although high CCNE1 mRNA expression was associated with relative resistance to palbociclib.
Figures




Comment in
-
Cyclin E mRNA: Assessing Cyclin-Dependent Kinase (CDK) Activation State to Elucidate Breast Cancer Resistance to CDK4/6 Inhibitors.J Clin Oncol. 2019 May 10;37(14):1148-1150. doi: 10.1200/JCO.19.00090. Epub 2019 Mar 28. J Clin Oncol. 2019. PMID: 30920879 Free PMC article. No abstract available.
References
-
- Ibrance (Palbociclib). Summary of Product Characteristics. Sandwich, Kent, UK, Pfizer, 2018.
-
- Ibrance (Palbociclib). Full Prescribing Information. New York, NY, Pfizer, 2018.
-
- Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. - PubMed
-
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;16:25–35. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

