A 12-Month Clinical Durability of Effectiveness and Safety Evaluation of a Vaginal Bowel Control System for the Nonsurgical Treatment of Fecal Incontinence
- PMID: 30807411
- PMCID: PMC6659396
- DOI: 10.1097/SPV.0000000000000681
A 12-Month Clinical Durability of Effectiveness and Safety Evaluation of a Vaginal Bowel Control System for the Nonsurgical Treatment of Fecal Incontinence
Abstract
Objective: The aim of this study was to characterize clinical success, impact on quality of life, and durability up to 1 year in women with fecal incontinence (FI) responsive to an initial test period with a trial vaginal bowel control system.
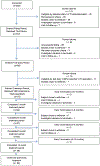
Methods: This was a prospective open-label study in subjects with FI and successfully fit who underwent an initial 2-week trial period. Those achieving 50% or greater reduction in FI episodes were provided the long-term system. Primary outcome was success at 3 months defined as 50% or greater reduction in baseline FI episodes, also assessed at 6 and 12 months. Secondary outcomes included symptom impact measured with Fecal Incontinence Quality of Life scale, symptom severity by the St Mark's (Vaizey) questionnaire, Patient Global Impression of Improvement, and satisfaction. Adverse events were collected. Primary analysis was intention to treat (ITT).
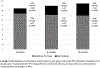
Results: Seventy-three subjects with baseline mean of 14.1 ± 12.15 FI episodes over 2 weeks entered the treatment period. Success rate at 3 months was 72.6% (53/73, P < 0.0001); per-protocol, 84.1% (53/63, P < 0.0001). Significant improvement in all Fecal Incontinence Quality of Life subscales and St Mark's questionnaire meeting minimally important differences was noted. Satisfaction was 91.7%, 89.7%, and 94.4% at 3, 6, and 12 months, respectively; 77.4%, 77.6%, and 79.6% were very much/much better on the Patient Global Impression of Improvement at 3, 6, and 12 months, respectively. Most common adverse event was vaginal wall injury, with most adverse events (90/134, 67%) occurring during fitting period.
Conclusions: In women with successful fitting and initial treatment response, durable efficacy was seen at 3, 6, and 12 months by objective and subjective measures, with favorable safety.
Trial registration: ClinicalTrials.gov NCT02428595.
Figures


References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

