Antenatal corticosteroid therapy (ACT) and size at birth: A population-based analysis using the Finnish Medical Birth Register
- PMID: 30807570
- PMCID: PMC6390995
- DOI: 10.1371/journal.pmed.1002746
Antenatal corticosteroid therapy (ACT) and size at birth: A population-based analysis using the Finnish Medical Birth Register
Abstract
Background: Antenatal corticosteroid therapy (ACT) is used clinically to prepare the fetal lung for impending preterm birth, but animal and human studies link corticosteroids to smaller birth size. Whether ACT is associated with birth size is debated; therefore, we assessed differences in birth size in treated versus untreated pregnancies.
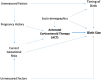
Methods and findings: This observational register-based study used data from the Finnish Medical Birth Register (FMBR) covering all births in Finland (January 1, 2006-December 31, 2010). We used unadjusted and adjusted regression analyses as well as propensity score matching (PSM) to analyze whether birth size differed by ACT exposure. PSM provides a stringent comparison, as subsamples were created matched on baseline and medical characteristics between treated and untreated women. All analyses were stratified by timing of birth. The primary study outcome was birth size: birth weight (BWT), birth length (BL), ponderal index (PI), and head circumference (HC) measured immediately after birth and recorded in the FMBR. Additional analyses explored indicators of neonatal health in relation to ACT exposure and birth size. A total of 278,508 live-born singleton births with ≥24 gestational completed weeks were registered in the FMBR during the 5-year study period. Over 4% of infants were born preterm, and 4,887 women were treated with ACT (1.75%). More than 44% of the exposed infants (n = 2,173) were born at term. First, results of unadjusted regression analyses using the entire sample showed the greatest reductions in BWT as compared to the other analytic methods: very preterm -61.26 g (±SE 24.12, P < 0.01), preterm -232.90 g (±SE 17.24, P < .001), near term -171.50 g (±SE 17.52, P < .001), and at term -101.95 g (±SE 10.89, P < .001). Second, using the entire sample, regression analyses adjusted for baseline and medical conditions showed significant differences in BWT between exposed and unexposed infants: very preterm -61.54 g (±SE 28.62, P < .03), preterm -222.78 g (±SE 19.64, P < .001), near term -159.25 g (±SE 19.14, P < .001), and at term -91.62 g (±SE 11.86, P < .03). Third, using the stringent PSM analyses based on matched subsamples, infants exposed to ACT weighed less at birth: -220.18 g (±SE 21.43, P < .001), -140.68 g (±SE 23.09, P < .001), and -89.38 g (±SE 14.16, P < .001), born preterm, near term, and at term, respectively. Similarly, significant reductions in BL and HC were also observed using the three analytic methods. There were no differences among postterm infants regardless of analytic method. Likewise, we observed no differences with respect to PI. Additional analyses showed that exposed and unexposed infants had generally similar Apgar scores at birth, yet the ACT-treated infants received greater medical care during the first 7 days of life and beyond. Our study is mainly limited by lack of data in FMBR specifying the interval between treatment and birth as well as other potential confounders that could not be tested.
Conclusions: In this study, ACT was consistently associated with reduction in birth size for infants born preterm, near term, or at term. Further investigation is warranted alongside reevaluation of guidelines. Efforts need to be made to correctly identify and target patients who will deliver preterm. Reduced growth should be considered when deliberating early care decisions.
Conflict of interest statement
The authors have declared that no competing interests exist. YW and AAK conducted this work while at Imperial College London and subsequently moved to Novartis Pharma AG, Basel, Switzerland, and Apple Tree Pediatrics, Karachi, Pakistan, respectively. Neither Novartis nor Apple Tree Pediatrics was involved in this research or has a financial stake in this work.
Figures
References
-
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3: 1465–1858. - PubMed
-
- Royal College of Obstetricians and Gynaecologists. Antenatal Corticosteroids to Reduce Neonatal Morbidity and Mortality. RCOG Green-top Guideline No. 7, 2010. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous