Sweet and bitter taste stimuli activate VTA projection neurons in the parabrachial nucleus
- PMID: 30807736
- PMCID: PMC6713447
- DOI: 10.1016/j.brainres.2019.02.027
Sweet and bitter taste stimuli activate VTA projection neurons in the parabrachial nucleus
Abstract
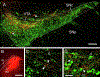
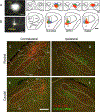
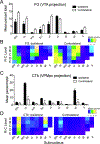
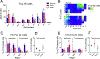
This study investigated neural projections from the parabrachial nucleus (PBN), a gustatory and visceral processing area in the brainstem, to the ventral tegmental area (VTA) in the midbrain. The VTA contains a large population of dopaminergic neurons that have been shown to play a role in reward processing. Anterograde neural tracing methods were first used to confirm that a robust projection from the caudal PBN terminates in the dorsal VTA; this projection was larger on the contralateral side. In the next experiment, we combined dual retrograde tracing from the VTA and the gustatory ventral posteromedial thalamus (VPMpc) with taste-evoked Fos protein expression, which labels activated neurons. Mice were stimulated through an intraoral cannula with sucrose, quinine, or water, and PBN sections were processed for immunofluorescent detection of Fos and retrograde tracers. The distribution of tracer-labeled PBN neurons demonstrated that the populations of cells projecting to the VTA or VPMpc are largely independent. Quantification of cells double labeled for Fos and either tracer demonstrated that sucrose and quinine were effective in activating both pathways. These results indicate that information about both appetitive and aversive tastes is delivered to a key midbrain reward interface via direct projections from the PBN.
Keywords: Fos; Neuroanatomy; Pathway; Reward; Taste; Tracer.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Activation of lateral hypothalamus-projecting parabrachial neurons by intraorally delivered gustatory stimuli.Front Neural Circuits. 2014 Jul 29;8:86. doi: 10.3389/fncir.2014.00086. eCollection 2014. Front Neural Circuits. 2014. PMID: 25120438 Free PMC article.
-
Mouse Parabrachial Neurons Signal a Relationship between Bitter Taste and Nociceptive Stimuli.J Neurosci. 2019 Feb 27;39(9):1631-1648. doi: 10.1523/JNEUROSCI.2000-18.2018. Epub 2019 Jan 3. J Neurosci. 2019. PMID: 30606758 Free PMC article.
-
Modulation of activity of gustatory neurons in the hamster parabrachial nuclei by electrical stimulation of the ventroposteromedial nucleus of the thalamus.Am J Physiol Regul Integr Comp Physiol. 2008 May;294(5):R1461-73. doi: 10.1152/ajpregu.00802.2007. Epub 2008 Mar 5. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18321954
-
VTA GABA Neurons at the Interface of Stress and Reward.Front Neural Circuits. 2019 Dec 5;13:78. doi: 10.3389/fncir.2019.00078. eCollection 2019. Front Neural Circuits. 2019. PMID: 31866835 Free PMC article. Review.
-
Ventral pallidal modulation of aversion processing.Brain Res. 2019 Jun 15;1713:62-69. doi: 10.1016/j.brainres.2018.10.010. Epub 2018 Oct 6. Brain Res. 2019. PMID: 30300634 Review.
Cited by
-
Neurobehavioral plasticity in the rodent gustatory system induced by regular consumption of a low-calorie sweetener during adolescence.Sci Rep. 2025 Jan 18;15(1):2359. doi: 10.1038/s41598-024-84391-3. Sci Rep. 2025. PMID: 39824856 Free PMC article.
-
Physiology of the tongue with emphasis on taste transduction.Physiol Rev. 2023 Apr 1;103(2):1193-1246. doi: 10.1152/physrev.00012.2022. Epub 2022 Nov 24. Physiol Rev. 2023. PMID: 36422992 Free PMC article. Review.
-
Cellular activity in insular cortex across seconds to hours: Sensations and predictions of bodily states.Neuron. 2021 Nov 17;109(22):3576-3593. doi: 10.1016/j.neuron.2021.08.036. Epub 2021 Sep 27. Neuron. 2021. PMID: 34582784 Free PMC article. Review.
-
Whole-brain efferent and afferent connectivity of mouse ventral tegmental area melanocortin-3 receptor neurons.J Comp Neurol. 2021 Apr 15;529(6):1157-1183. doi: 10.1002/cne.25013. Epub 2020 Sep 10. J Comp Neurol. 2021. PMID: 32856297 Free PMC article.
-
Experience-dependent changes in affective valence of taste in male mice.Mol Brain. 2023 Mar 11;16(1):28. doi: 10.1186/s13041-023-01017-x. Mol Brain. 2023. PMID: 36906607 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

