Adrenal androgens rescue prostatic dihydrotestosterone production and growth of prostate cancer cells after castration
- PMID: 30807787
- PMCID: PMC6438375
- DOI: 10.1016/j.mce.2019.02.018
Adrenal androgens rescue prostatic dihydrotestosterone production and growth of prostate cancer cells after castration
Abstract
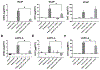
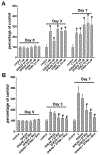
Adrenal androgens dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) are potential substrates for intracrine production of testosterone (T) and dihydrotestosterone (DHT), or directly to DHT, by prostate cancer (PCa) cells. Production of DHT from DHEAS and DHEA, and the role of steroid sulfatase (STS), were evaluated ex vivo using fresh human prostate tissue and in vitro using human PCa cell lines. STS was expressed in benign prostate tissue and PCa tissue. DHEAS at a physiological concentration was converted to DHT in prostate tissue and PCa cell lines, which was STS-dependent. DHEAS activation of androgen receptor (AR) and stimulation of PCa cell growth were STS-dependent. DHEA at a physiological concentration was not converted to DHT ex vivo and in vitro, but stimulated in vivo tumor growth of the human PCa cell line, VCaP, in castrated mice. The findings suggest that targeting metabolism of DHEAS and DHEA may enhance androgen deprivation therapy.
Keywords: Adrenal androgen; Androgen; Intracrine androgen metabolism; Metabolism; Prostate cancer; Steroid sulfatase.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest: The authors declare no potential conflicts of interest.
Figures


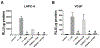

References
-
- Wilson EM and French FS, 1976. Binding properties of androgen receptors. Evidence for identical receptors in rat testis, epididymis, and prostate, J Biol Chem 251, 5620–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

