Cortical Bone Derived Stem Cells for Cardiac Wound Healing
- PMID: 30808081
- PMCID: PMC6428954
- DOI: 10.4070/kcj.2018.0437
Cortical Bone Derived Stem Cells for Cardiac Wound Healing
Abstract
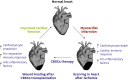
Ischemic heart disease can lead to myocardial infarction (MI), a major cause of morbidity and mortality worldwide. Adoptive transfer of multiple stem cell types into failing human hearts has demonstrated safety however the beneficial effects in patients with cardiovascular disorders have been modest. Modest improvement in patients with cardiac complications warrants identification of a novel stem cell population that possesses effective reparative properties and improves cardiac function after injury. Recently we have shown in a mouse model and a porcine pre-clinical animal model, that cortical bone derived stem cells (CBSCs) enhance cardiac function after MI and/or ischemia-reperfusion injury. These beneficial effects of allogeneic cell delivery appear to be mediated by paracrine mechanisms rather than by transdifferentiation of injected cells into vessels and/or immature myocytes. This review will discuss role of CBSCs in cardiac wound healing. After having modest beneficial improvement in most of the clinical trials, a critical need is to understand the interaction of the transplanted stem cells with the ischemic cardiac environment. Transplanted stem cells are exposed to pro-inflammatory factors and activated immune cells and fibroblasts, but their interactions remain unknown. We have shown that CBSCs modulate different processes including modulation of the immune response, angiogenesis, and restriction of infarct sizes after cardiac injury. This review will provide information on unique protective signature of CBSCs in rodent/swine animal models for heart repair that should provide basis for developing novel therapies for treating heart failure patients.
Keywords: Cell therapy; Fibrosis; Immunomodulation; Myocardial infarction; Wound healing.
Copyright © 2019. The Korean Society of Cardiology.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
Similar articles
-
Cortical bone-derived stem cell therapy reduces apoptosis after myocardial infarction.Am J Physiol Heart Circ Physiol. 2019 Oct 1;317(4):H820-H829. doi: 10.1152/ajpheart.00144.2019. Epub 2019 Aug 23. Am J Physiol Heart Circ Physiol. 2019. PMID: 31441690 Free PMC article.
-
Cortical bone stem cells modify cardiac inflammation after myocardial infarction by inducing a novel macrophage phenotype.Am J Physiol Heart Circ Physiol. 2021 Oct 1;321(4):H684-H701. doi: 10.1152/ajpheart.00304.2021. Epub 2021 Aug 20. Am J Physiol Heart Circ Physiol. 2021. PMID: 34415185 Free PMC article.
-
Unique Features of Cortical Bone Stem Cells Associated With Repair of the Injured Heart.Circ Res. 2015 Dec 4;117(12):1024-33. doi: 10.1161/CIRCRESAHA.115.307362. Epub 2015 Oct 15. Circ Res. 2015. PMID: 26472818
-
Healing the Broken Heart; The Immunomodulatory Effects of Stem Cell Therapy.Front Immunol. 2020 Apr 9;11:639. doi: 10.3389/fimmu.2020.00639. eCollection 2020. Front Immunol. 2020. PMID: 32328072 Free PMC article. Review.
-
The Regulatory Role of T Cell Responses in Cardiac Remodeling Following Myocardial Infarction.Int J Mol Sci. 2020 Jul 16;21(14):5013. doi: 10.3390/ijms21145013. Int J Mol Sci. 2020. PMID: 32708585 Free PMC article. Review.
Cited by
-
Cortical bone stem cell-derived exosomes' therapeutic effect on myocardial ischemia-reperfusion and cardiac remodeling.Am J Physiol Heart Circ Physiol. 2021 Dec 1;321(6):H1014-H1029. doi: 10.1152/ajpheart.00197.2021. Epub 2021 Oct 8. Am J Physiol Heart Circ Physiol. 2021. PMID: 34623184 Free PMC article.
-
Main histological parameters to be evaluated in an experimental model of myocardial infarct treated by stem cells on pigs.PeerJ. 2019 Jul 22;7:e7160. doi: 10.7717/peerj.7160. eCollection 2019. PeerJ. 2019. PMID: 31367480 Free PMC article.
-
Stem Cell and Exosome Therapy in Pulmonary Hypertension.Korean Circ J. 2022 Feb;52(2):110-122. doi: 10.4070/kcj.2021.0191. Korean Circ J. 2022. PMID: 35128849 Free PMC article. Review.
-
Hemovac blood after total knee arthroplasty as a source of stem cells.Ann Transl Med. 2020 Nov;8(21):1406. doi: 10.21037/atm-20-2215. Ann Transl Med. 2020. PMID: 33313151 Free PMC article.
-
Melatonin attenuates radiation-induced cortical bone-derived stem cells injury and enhances bone repair in postradiation femoral defect model.Mil Med Res. 2021 Dec 12;8(1):61. doi: 10.1186/s40779-021-00355-y. Mil Med Res. 2021. PMID: 34895335 Free PMC article.
References
-
- Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. - PubMed
-
- Gerdes AM. Cardiac myocyte remodeling in hypertrophy and progression to failure. J Card Fail. 2002;8:S264–S268. - PubMed
-
- Loughran JH, Chugh AR, Ismail I, Bolli R. Stem cell therapy: promising treatment in heart failure? Curr Heart Fail Rep. 2013;10:73–80. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources