The impact of local control on overall survival after stereotactic body radiotherapy for liver and lung metastases from colorectal cancer: a combined analysis of 388 patients with 500 metastases
- PMID: 30808323
- PMCID: PMC6390357
- DOI: 10.1186/s12885-019-5362-5
The impact of local control on overall survival after stereotactic body radiotherapy for liver and lung metastases from colorectal cancer: a combined analysis of 388 patients with 500 metastases
Abstract
Background: The aim of this analysis was to model the effect of local control (LC) on overall survival (OS) in patients treated with stereotactic body radiotherapy (SBRT) for liver or lung metastases from colorectal cancer.
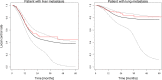
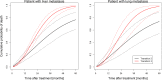
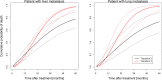
Methods: The analysis is based on pooled data from two retrospective SBRT databases for pulmonary and hepatic metastases from 27 centers from Germany and Switzerland. Only patients with metastases from colorectal cancer were considered to avoid histology as a confounding factor. An illness-death model was employed to model the relationship between LC and OS.
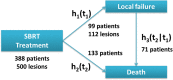
Results: Three hundred eighty-eight patients with 500 metastatic lesions (lung n = 209, liver n = 291) were included and analyzed. Median follow-up time for local recurrence assessment was 12.1 months. Ninety-nine patients with 112 lesions experienced local failure. Seventy-one of these patients died after local failure. Median survival time was 27.9 months in all patients and 25.4 months versus 30.6 months in patients with and without local failure after SBRT. The baseline risk of death after local failure exceeds the baseline risk of death without local failure at 10 months indicating better survival with LC.
Conclusion: In CRC patients with lung or liver metastases, our findings suggest improved long-term OS by achieving metastatic disease control using SBRT in patients with a projected OS estimate of > 12 months.
Keywords: Colorectal cancer; Illness-death model; Liver metastases; Lung metastases; Stereotactic body radiation therapy; Tumor control probability.
Conflict of interest statement
Ethics approval and consent to participate
The multicenter data collection and analysis was approved by the Ethics committee of the Kanton Zurich, Switzerland (BASEC-Nr. 2016–00744) and in addition to local regulations also covered the following institutions:
University Hospital Zürich, Department of Radiation Oncology, University of Zurich, Zurich, Switzerland.
Strahlentherapie Bautzen, Department of Radiation Oncology, Bautzen, Germany
University of Munich – LMU Munich, Department of Radiation Oncology,Munich, German
University Hospital Basel, Department of Radiation Oncology, Basel, Switzerland
University Medical Center Hamburg-Eppendorf, Department of Radiation Oncology, Hamburg, Germany
Strahlenzentrum Hamburg, Department of Radiation Oncology, Hamburg, Germany
University Hospital of Cologne, Department of Radiation Oncology, Cologne, Germany
University Hospital Würzburg, Department of Radiation Oncology, Würzburg, Germany
University Hospital Halle, Department of Radiation Oncology, Halle, Germany
Klinikum Passau, Radiation Oncology, Passau, Germany
If necessary, the data collection of the individual participating centers was approved according to local regulations and approved by the respective local ethics committees. The following ethics committees and regulatory bodies were involved in this local approval process:
Medizinische Ethik-Komission II, Medizinische Fakultät Mannheim; 2014-413 M-MA-§23bMPG: University Hospital Mannheim, Department of Radiation Oncology, University of Heidelberg, Mannheim, Germany.
Ethikkommission der Medizinischen Fakultät Heidelberg; S459–2010:
Ethikkommission der Medizinischen Fakultät der Technischen Universität München; 84/16S: Klinikum rechts der Isar- Technische Universität München, Department of Radiation Oncology, Munich, Germany
Ethikkommission an der Medizinischen Fakultät der Universität Rostock, A2016–0008:
Universitätsklinikum Schleswig-Holstein, Department of Radiation Oncology, Kiel/Lübeck, Germany.
University Hospital Rostock, Department of Radiation Oncology, Rostock, Germany.
Ethikkommission der Universität Freiburg, 462/12: University Hospital Freiburg, Department of Radiation Oncology, Freiburg, Germany
Ärztekammer: Bezirksärztekammer Nord-Württemberg, Jahnstr. 5, 70,597 Stuttgart: RadioChirurgicum CyberKnife Südwest, Radiation Oncology, Göppingen, Germany.
Ethikkommission der Bayerischen Ärztekammer, mb BO 16002: Krankenhaus Barmherzige Brüder, Department of Radiation Oncology, Regensburg, Germany
The participants consent was written as part of the main ethics approval.
Consent for publication
Not applicable.
Competing interests
Marciana Duma and Christian Ostheimer are members of the editorial board (Associate editors) of BMC Cancer. NA confirms that all other authors have nothing to declare at the time of submission and that there are no competing interests to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–91. 10.1136/gutjnl-2015-310912. - PubMed
-
- Ahmed KA, Fulp WJ, Berglund AE, Hoffe SE, Dilling TJ, Eschrich SA, et al. Differences between Colon Cancer primaries and metastases using a molecular assay for tumor radiation sensitivity suggest implications for potential Oligometastatic SBRT patient selection. Int J Radiat Oncol Biol Phys. 2015;92:837–842. doi: 10.1016/j.ijrobp.2015.01.036. - DOI - PMC - PubMed

