Mixed-methods approach to evaluate an mHealth intervention to increase adherence to triage of human papillomavirus-positive women who have performed self-collection (the ATICA study): study protocol for a hybrid type I cluster randomized effectiveness-implementation trial
- PMID: 30808379
- PMCID: PMC6390557
- DOI: 10.1186/s13063-019-3229-3
Mixed-methods approach to evaluate an mHealth intervention to increase adherence to triage of human papillomavirus-positive women who have performed self-collection (the ATICA study): study protocol for a hybrid type I cluster randomized effectiveness-implementation trial
Abstract
Background: Cervical cancer is one of the leading causes of cancer death among women worldwide, with more than 85% of cases occurring in low- and middle-income countries. Human papillomavirus (HPV) screening allows for self-collection with the potential to increase coverage, but still requires triage to identify which HPV+ women need diagnostic and treatment procedures. However, achieving high levels of triage adherence can be challenging, especially among socially vulnerable women. This paper describes the ATICA protocol (Application of Communication and Information Technologies to Self-Collection, for its initials in Spanish), aimed at evaluating the implementation strategy and the effectiveness of a multi-component mobile health (mHealth) intervention to increase adherence to triage among women with HPV+ self-collected tests.
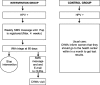
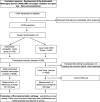
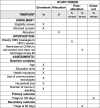
Methods: We will use an effectiveness-implementation hybrid type I trial with a mixed-methods evaluation approach. A cluster randomized trial design including 200 community health workers (CHWs) will evaluate whether the mHealth intervention increases adherence to triage among HPV+ women who self-collected at home during a CHW visit within 120 days after a positive result. The intervention includes an initial mobile phone text message (SMS) alert and subsequent reminders sent to HPV+ women. For those who do not adhere to triage within 60 days of a positive HPV test, an email and SMS will be sent to the CHWs to promote contact with these women during home visits. We will use the Consolidated Framework for Implementation Research (CFIR) as an organizing and analytic framework to evaluate the implementation of the intervention while also drawing on Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM). We will conduct a self-administered, semi-structured survey of CHWs, semi-structured interviews with local health authorities, and a survey of HPV+ women. Combining both qualitative and quantitative data will provide rich insights into local implementation challenges and successes.
Discussion: Findings from the implementation evaluation will be applicable to programs that use or are planning to incorporate HPV self-collection and/or mHealth interventions in different settings and countries. This innovative study will also serve as a model for using implementation science in the region.
Trial registration: ClinicalTrials.gov, NCT03478397 . Registered on 20 March 2018.
Keywords: Community health workers; HPV; Implementation; Self-collection; mHealth.
Conflict of interest statement
Ethics approval and consent to participate
This protocol was approved by the CEMIC Institutional Review Board, the Ethics Research Committee of the Jujuy Ministry of Health, The Institutional Review Board of the Harvard T.H. Chan School of Public Health and the Deakin University Human Research Ethics Committee. The trial is registered in ClinicalTrials.gov (NCT03478397) and the Deakin University Human Ethics Research Committee (APP 2018–039). Women have to sign an informed consent before the study begins, as well as CHWs participating in the CHW survey. Anonymity of participants is guaranteed.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008. 10.1016/j.vaccine.2008.06.019. - PubMed
-
- Arbyn M, Verdoodt F, Snijders PJ, Verthoef VM, Suonio E, Dillner L, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014. 10.1016/S1470-2045(13)70570-9. - PubMed
-
- Lazcano-Ponce E, Lorincz AT, Cruz-Valdez A, Salmerón J, Uribe P, Velasco-Mondragón E, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011. 10.1016/S0140-6736(11)61522-5. - PubMed
-
- Cuschieri K, Ronco G, Lorincz A, Smith L, Ogilvie G, Mirabello L, Carozzi F, Cubie H, Wentzensen N, Snijders P, Arbyn M, Monsonego J, Franceschi S. Eurogin roadmap 2017: triage strategies for the management of HPV-positive women in cervical screening programs. Int J Cancer. 2018. 10.1002/ijc.31261. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

