The efficacy and safety of velagliflozin over 16 weeks as a treatment for insulin dysregulation in ponies
- PMID: 30808423
- PMCID: PMC6390376
- DOI: 10.1186/s12917-019-1811-2
The efficacy and safety of velagliflozin over 16 weeks as a treatment for insulin dysregulation in ponies
Abstract
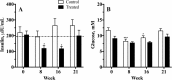
Background: A previous six-week (wk) study demonstrated the potential of the sodium-glucose linked transport inhibitor velagliflozin as a novel treatment for equine insulin dysregulation. The present study examined the safety and efficacy of velagliflozin over 16 wk. of treatment, and over 4 wk. of withdrawal. Twenty-four insulin dysregulated ponies were selected, based on their hyper-responsiveness to a diet challenge meal containing 3.8 g non-structural carbohydrates (NSC)/kg bodyweight (BW). Ponies with serum insulin > 90 μIU/mL either 2 or 4 h after feeding were enrolled, and randomly allocated to receive either velagliflozin (0.3 mg/kg BW orally once daily, n = 12), or a placebo (n = 10-12) for 16 wk. The subjects were fed 7.5 g NSC/kg BW/day to maintain a fat body condition. Safety was assessed through daily monitoring, veterinary examination, and the measurement of fasting blood glucose, biochemistry and haematology. Efficacy at reducing post-prandial hyperinsulinemia was assessed using a diet challenge every 8 wk. during treatment and 4 wk. after withdrawal.
Results: Velagliflozin was well accepted by all subjects and caused no adverse effects or hypoglycaemia. Post-prandial serum insulin (insulin Cmax) did not change significantly in the control animals over the entire study period (P = 0.101). In contrast, insulin Cmax (mean ± SE) concentrations fell over time in the velagliflozin-treated group from 205 ± 25 μIU/mL in wk. 0, to 119 ± 19 μIU/mL (P = 0.015) and 117 ± 15 μIU/ml (P = 0.029) after 8 and 16 wk. of treatment, respectively. Although the insulin Cmax in this group was not significantly lower than in controls at wk-8 (P = 0.061), it was lower at wk-16 (P = 0.003), and all 12 treated ponies were below the previously-determined risk threshold for laminitis at this time. After 4 wk. withdrawal, the insulin Cmax returned to 199 ± 36 μIU/mL in the treated group, with no rebound effect.
Conclusions: Velagliflozin appears to be a promising and safe treatment for equine insulin dysregulation, bringing post-prandial insulin concentrations below the laminitis risk threshold, albeit without normalising them.
Keywords: Equine metabolic syndrome; Insulin; Laminitis; Sodium-glucose linked transport inhibitor.
Conflict of interest statement
Ethics approval
This study was approved by the Animal Care and Ethics Committees of Queensland University of Technology (1500000204) and The University of Queensland (QUT/SVS/470/14). All procedures were conducted in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes (NHMRC, 8th edition, 2013). All animals used in the study were owned by Queensland University of Technology and were used with the consent of the University.
Consent for publication
Not applicable.
Competing interests
DR is an employee of the company that funded this research: Boehringer-Ingelheim.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
The sodium-glucose co-transporter 2 inhibitor velagliflozin reduces hyperinsulinemia and prevents laminitis in insulin-dysregulated ponies.PLoS One. 2018 Sep 13;13(9):e0203655. doi: 10.1371/journal.pone.0203655. eCollection 2018. PLoS One. 2018. PMID: 30212530 Free PMC article.
-
The oral glucose test predicts laminitis risk in ponies fed a diet high in nonstructural carbohydrates.Domest Anim Endocrinol. 2018 Apr;63:1-9. doi: 10.1016/j.domaniend.2017.10.008. Epub 2017 Nov 16. Domest Anim Endocrinol. 2018. PMID: 29172109
-
The effect of different grazing conditions on the insulin and incretin response to the oral glucose test in ponies.BMC Vet Res. 2019 Oct 16;15(1):345. doi: 10.1186/s12917-019-2088-1. BMC Vet Res. 2019. PMID: 31619223 Free PMC article.
-
Sodium-glucose transport protein 2 inhibitor use in the management of insulin dysregulation in ponies and horses.J Vet Pharmacol Ther. 2025 Jan;48 Suppl 1(Suppl 1):31-40. doi: 10.1111/jvp.13470. Epub 2024 Jul 10. J Vet Pharmacol Ther. 2025. PMID: 38984777 Free PMC article. Review.
-
Insulin dysregulation.Equine Vet J. 2014 Jan;46(1):103-12. doi: 10.1111/evj.12169. Epub 2013 Nov 18. Equine Vet J. 2014. PMID: 24033478 Review.
Cited by
-
Use of the SGLT2 inhibitor canagliflozin for control of refractory equine hyperinsulinemia and laminitis.Open Vet J. 2022 Jul-Aug;12(4):511-518. doi: 10.5455/OVJ.2022.v12.i4.14. Epub 2022 Aug 7. Open Vet J. 2022. PMID: 36118716 Free PMC article.
-
Hypertriglyceridemia in equines with refractory hyperinsulinemia treated with SGLT2 inhibitors.Open Vet J. 2023 Mar;13(3):365-375. doi: 10.5455/OVJ.2023.v13.i3.14. Epub 2023 Mar 20. Open Vet J. 2023. PMID: 37026076 Free PMC article.
-
Effect of sirolimus on insulin dynamics in horses.J Vet Intern Med. 2023 Mar;37(2):703-712. doi: 10.1111/jvim.16650. Epub 2023 Feb 25. J Vet Intern Med. 2023. PMID: 36840433 Free PMC article.
-
Pharmacokinetics and Alterations in Glucose and Insulin Levels After a Single Dose of Canagliflozin in Healthy Icelandic Horses.J Vet Pharmacol Ther. 2025 Jan;48 Suppl 1(Suppl 1):41-49. doi: 10.1111/jvp.13476. Epub 2024 Aug 7. J Vet Pharmacol Ther. 2025. PMID: 39113254 Free PMC article.
-
Short-term effects of canagliflozin on glucose and insulin responses in insulin dysregulated horses: A randomized, placebo-controlled, double-blind, study.J Vet Intern Med. 2023 Nov-Dec;37(6):2520-2528. doi: 10.1111/jvim.16906. Epub 2023 Oct 21. J Vet Intern Med. 2023. PMID: 37864426 Free PMC article.
References
-
- Frank N, Tadros E. Insulin dysregulation. Equine Vet J. 2014;46(1):103–112. - PubMed
-
- Asplin K, Sillence M, Pollitt C, McGowan C. Induction of laminitis by prolonged hyperinsulinaemia in clinically normal ponies. Vet J. 2007;174(3):530–535. - PubMed
-
- de Laat M, McGowan C, Sillence M, Pollitt C. Equine laminitis: induced by 48 h hyperinsulinaemia in Standardbred horses. Equine Vet J. 2010;42(2):129–135. - PubMed
-
- Meier A, de Laat M, Reiche D, Pollitt C, Walsh D, McGree J, Sillence M. The oral glucose test predicts laminitis risk in ponies fed a diet high in nonstructural carbohydrates. Domest Anim Endocrinol. 2018;63:1–9. - PubMed
-
- Menzies-Gow N, Harris P, Elliott J. Prospective cohort study evaluating risk factors for the development of pasture-associated laminitis in the United Kingdom. Equine Vet J. 2016;49(3):300–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

