PTPN14 phosphatase and YAP promote TGFβ signalling in rheumatoid synoviocytes
- PMID: 30808624
- PMCID: PMC7039277
- DOI: 10.1136/annrheumdis-2018-213799
PTPN14 phosphatase and YAP promote TGFβ signalling in rheumatoid synoviocytes
Abstract
Objective: We aimed to understand the role of the tyrosine phosphatase PTPN14-which in cancer cells modulates the Hippo pathway by retaining YAP in the cytosol-in fibroblast-like synoviocytes (FLS) from patients with rheumatoid arthritis (RA).
Methods: Gene/protein expression levels were measured by quantitative PCR and/or Western blotting. Gene knockdown in RA FLS was achieved using antisense oligonucleotides. The interaction between PTPN14 and YAP was assessed by immunoprecipitation. The cellular localisation of YAP and SMAD3 was examined via immunofluorescence. SMAD reporter studies were carried out in HEK293T cells. The RA FLS/cartilage coimplantation and passive K/BxN models were used to examine the role of YAP in arthritis.
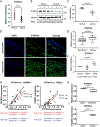
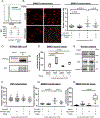
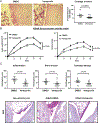
Results: RA FLS displayed overexpression of PTPN14 when compared with FLS from patients with osteoarthritis (OA). PTPN14 knockdown in RA FLS impaired TGFβ-dependent expression of MMP13 and potentiation of TNF signalling. In RA FLS, PTPN14 formed a complex with YAP. Expression of PTPN14 or nuclear YAP-but not of a non-YAP-interacting PTPN14 mutant-enhanced SMAD reporter activity. YAP promoted TGFβ-dependent SMAD3 nuclear localisation in RA FLS. Differences in epigenetic marks within Hippo pathway genes, including YAP, were found between RA FLS and OA FLS. Inhibition of YAP reduced RA FLS pathogenic behaviour and ameliorated arthritis severity.
Conclusion: In RA FLS, PTPN14 and YAP promote nuclear localisation of SMAD3. YAP enhances a range of RA FLS pathogenic behaviours which, together with epigenetic evidence, points to the Hippo pathway as an important regulator of RA FLS behaviour.
Keywords: K/BxN; PTPN14; TGFβ; Verteporfin; YAP; fibroblast-like synoviocytes; rheumatoid arthritis.
© Author(s) (or their employer(s)) 2019. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
circ_0002970 promotes fibroblast-like synoviocytes invasion and the inflammatory response through Hippo/YAP signaling to induce CTGF/CCN1 expression in rheumatoid arthritis.Arthritis Res Ther. 2025 Apr 25;27(1):97. doi: 10.1186/s13075-025-03562-3. Arthritis Res Ther. 2025. PMID: 40281642 Free PMC article.
-
Knockdown of YAP/TAZ Inhibits the Migration and Invasion of Fibroblast Synovial Cells in Rheumatoid Arthritis by Regulating Autophagy.J Immunol Res. 2020 Oct 22;2020:9510594. doi: 10.1155/2020/9510594. eCollection 2020. J Immunol Res. 2020. PMID: 33145365 Free PMC article.
-
TGFβ responsive tyrosine phosphatase promotes rheumatoid synovial fibroblast invasiveness.Ann Rheum Dis. 2016 Jan;75(1):295-302. doi: 10.1136/annrheumdis-2014-205790. Epub 2014 Nov 6. Ann Rheum Dis. 2016. PMID: 25378349 Free PMC article.
-
Role of glucose metabolism in aggressive phenotype of fibroblast-like synoviocytes: Latest evidence and therapeutic approaches in rheumatoid arthritis.Int Immunopharmacol. 2020 Dec;89(Pt A):107064. doi: 10.1016/j.intimp.2020.107064. Epub 2020 Oct 8. Int Immunopharmacol. 2020. PMID: 33039953 Review.
-
E3 ubiquitin ligase gene BIRC3 modulates TNF-induced cell death pathways and promotes aberrant proliferation in rheumatoid arthritis fibroblast-like synoviocytes.Front Immunol. 2024 Sep 5;15:1433898. doi: 10.3389/fimmu.2024.1433898. eCollection 2024. Front Immunol. 2024. PMID: 39301019 Free PMC article. Review.
Cited by
-
Identification of immunological characterization and Anoikis-related molecular clusters in rheumatoid arthritis.Front Mol Biosci. 2023 Nov 17;10:1202371. doi: 10.3389/fmolb.2023.1202371. eCollection 2023. Front Mol Biosci. 2023. PMID: 38046810 Free PMC article.
-
Adipose-derived stem cells postpone the progression of Sjögren's syndrome by upregulating the Hippo signaling pathway.Exp Ther Med. 2022 Jul 22;24(3):587. doi: 10.3892/etm.2022.11524. eCollection 2022 Sep. Exp Ther Med. 2022. PMID: 35949326 Free PMC article.
-
YAP/TAZ: Key Players for Rheumatoid Arthritis Severity by Driving Fibroblast Like Synoviocytes Phenotype and Fibro-Inflammatory Response.Front Immunol. 2021 Dec 9;12:791907. doi: 10.3389/fimmu.2021.791907. eCollection 2021. Front Immunol. 2021. PMID: 34956224 Free PMC article.
-
MicroRNA-21 from bone marrow mesenchymal stem cell-derived extracellular vesicles targets TET1 to suppress KLF4 and alleviate rheumatoid arthritis.Ther Adv Chronic Dis. 2021 Apr 24;12:20406223211007369. doi: 10.1177/20406223211007369. eCollection 2021. Ther Adv Chronic Dis. 2021. PMID: 33995992 Free PMC article.
-
The Hippo signalling pathway in bone homeostasis: Under the regulation of mechanics and aging.Cell Prolif. 2024 Oct;57(10):e13652. doi: 10.1111/cpr.13652. Epub 2024 May 3. Cell Prolif. 2024. PMID: 38700015 Free PMC article. Review.
References
-
- Feldmann M, Maini RN. Perspectives From Masters in Rheumatology and Autoimmunity: Can We Get Closer to a Cure for Rheumatoid Arthritis? Arthritis Rheumatol. 2015. September; 67(9):2283–2291. - PubMed

