Transfusion-related red blood cell alloantibodies: induction and consequences
- PMID: 30808636
- PMCID: PMC6484385
- DOI: 10.1182/blood-2018-08-833962
Transfusion-related red blood cell alloantibodies: induction and consequences
Abstract
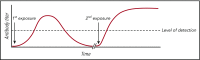
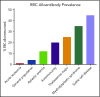
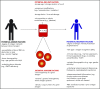
Blood transfusion is the most common procedure completed during a given hospitalization in the United States. Although often life-saving, transfusions are not risk-free. One sequela that occurs in a subset of red blood cell (RBC) transfusion recipients is the development of alloantibodies. It is estimated that only 30% of induced RBC alloantibodies are detected, given alloantibody induction and evanescence patterns, missed opportunities for alloantibody detection, and record fragmentation. Alloantibodies may be clinically significant in future transfusion scenarios, potentially resulting in acute or delayed hemolytic transfusion reactions or in difficulty locating compatible RBC units for future transfusion. Alloantibodies can also be clinically significant in future pregnancies, potentially resulting in hemolytic disease of the fetus and newborn. A better understanding of factors that impact RBC alloantibody formation may allow general or targeted preventative strategies to be developed. Animal and human studies suggest that blood donor, blood product, and transfusion recipient variables potentially influence which transfusion recipients will become alloimmunized, with genetic as well as innate/adaptive immune factors also playing a role. At present, judicious transfusion of RBCs is the primary strategy invoked in alloimmunization prevention. Other mitigation strategies include matching RBC antigens of blood donors to those of transfusion recipients or providing immunomodulatory therapies prior to blood product exposure in select recipients with a history of life-threatening alloimmunization. Multidisciplinary collaborations between providers with expertise in transfusion medicine, hematology, oncology, transplantation, obstetrics, and immunology, among other areas, are needed to better understand RBC alloimmunization and refine preventative strategies.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- US Department of Health and Human Services (FDA). Fatalities Reported to the FDA Following Blood Collection. http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability. Accessed 1 August 2018.
-
- Yazer MH, Jackson B, Beckman N, et al. ; Biomedical Excellence for Safer Transfusions (BEST) Collaborative. Changes in blood center red blood cell distributions in the era of patient blood management: the trends for collection (TFC) study. Transfusion. 2016;56(8):1965-1973. - PubMed
-
- Reid ME, Mohandas N. Red blood cell blood group antigens: structure and function. Semin Hematol. 2004;41(2):93-117. - PubMed
-
- Tormey CA, Fisk J, Stack G. Red blood cell alloantibody frequency, specificity, and properties in a population of male military veterans. Transfusion. 2008;48(10):2069-2076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

