Effectiveness and safety of nicotine patches combined with e-cigarettes (with and without nicotine) for smoking cessation: study protocol for a randomised controlled trial
- PMID: 30808668
- PMCID: PMC6398670
- DOI: 10.1136/bmjopen-2018-023659
Effectiveness and safety of nicotine patches combined with e-cigarettes (with and without nicotine) for smoking cessation: study protocol for a randomised controlled trial
Abstract
Introduction: Evidence indicates e-cigarettes can help people quit smoking; however, more confirmatory trials are needed. To date, no trials have evaluated the effectiveness and safety of combining nicotine patches with e-cigarettes (with and without nicotine) for smoking cessation.
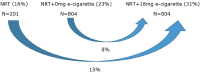
Methods and analysis: This study is a pragmatic, three-arm, community-based, single-blind, randomised trial undertaken in New Zealand. Eligible participants are daily/non-daily smokers, aged ≥18 years, naive e-cigarette users and motivated to quit smoking in the next 2 weeks. Participants (n=1809), recruited using multi-media advertising, are randomised to 14 weeks of (1) 21 mg nicotine patches (n=201); (2) 21 mg nicotine patches+18 mg/mL nicotine e-cigarette (n=804); or (3) 21 mg nicotine patches+nicotine free e-cigarette (n=804). Participants receive weekly withdrawal-oriented behavioural support calls for 6 weeks post-randomisation. The primary outcome is self-reported biochemically verified continuous abstinence (CA) at 6 months post quit-date. The primary comparison is nicotine patch + nicotine e-cigarette versus nicotine patch + nicotine free e-cigarette, and the secondary comparison is nicotine patch versus nicotine patch +nicotine e-cigarette (90% power, p=0.05, to detect an absolute difference in 6 month CA rates of 8% and 15% respectively). Secondary outcomes, collected by phone interview at quit date, then 1, 3, 6 and 12 months post-quit date, include self-reported CA, 7 day point prevalence abstinence, cigarettes per day (if smoking, or when smoking for non-daily smokers), time to relapse (if returned to smoking), belief in ability to quit, use of other cessation support, side effects/serious adverse events, treatment compliance, seeking additional support around e-cigarette use, daily use of both e-cigarettes and cigarettes, use of treatment past 14 weeks, views on treatment and recommendation to others, weight and cost-per-quitter.
Ethics and dissemination: The Northern A Health and Disability Ethics Committee approved the trial. Findings will be disseminated through publication, conference/meeting presentations, and media.
Trial registration number: NCT02521662; Pre-results.
Keywords: cessation; e-cigarettes; effectiveness; electronic cigarettes; nicotine patch; randomised controlled trial; safety.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No authors have received financial support for the submitted work from any companies with a financial interest in the products under investigation. CB has received benefits in kind (accommodation expenses) from a manufacturer of smoking cessation medications. NW has provided consultancy to the manufacturers of smoking cessation medications, received honoraria for speaking at a research meeting and received benefits in kind and travel support from a manufacturer of smoking cessation medications (but over five year ago). NW, CB, MV, GL and VP are currently involved in a clinical trial in which varenicline and matching placebo are supplied by Pfizer under their Investigator-Initiated Research Program. MV has previously undertaken research supported by an unrestricted grant from Pfizer. None of the authors’ spouses, partners or children have financial relationships that may be relevant to the submitted work. All authors have no non-financial interests that may be relevant to the submitted work.
Figures
References
-
- Przulj D, McRobbie H. The effect of sensorimotor replacement on smoking cessation and craving. Open Addict J 2012;5:41–50. 10.2174/1874941001205010041 - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous