Antibody-Mediated Endocytosis of Polysialic Acid Enables Intracellular Delivery and Cytotoxicity of a Glycan-Directed Antibody-Drug Conjugate
- PMID: 30808675
- PMCID: PMC6467748
- DOI: 10.1158/0008-5472.CAN-18-3119
Antibody-Mediated Endocytosis of Polysialic Acid Enables Intracellular Delivery and Cytotoxicity of a Glycan-Directed Antibody-Drug Conjugate
Abstract
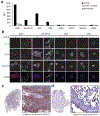
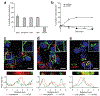
The specific targeting of differentially expressed glycans in malignant cells has emerged as an attractive anticancer strategy. One such target is the oncodevelopmental antigen polysialic acid (polySia), a polymer of α2,8-linked sialic acid residues that is largely absent during postnatal development but is re-expressed during progression of several malignant human tumors, including small-cell and non-small cell lung carcinomas, glioma, neuroblastoma, and pancreatic carcinoma. In these cancers, expression of polySia correlates with tumor progression and poor prognosis and appears to modulate cancer cell adhesion, invasiveness, and metastasis. To evaluate the potential of PolySia as a target for anticancer therapy, we developed a chimeric human polySia-specific mAb that retained low nanomolar (nmol/L) target affinity and exhibited exquisite selectivity for polySia structures. The engineered chimeric mAb recognized several polySia-positive tumor cell lines in vitro and induced rapid endocytosis of polySia antigens. To determine whether this internalization could be exploited for delivery of conjugated cytotoxic drugs, we generated an antibody-drug conjugate (ADC) by covalently linking the chimeric human mAb to the tubulin-binding maytansinoid DM1 using a bioorthogonal chemical reaction scheme. The resulting polySia-directed ADC demonstrated potent target-dependent cytotoxicity against polySia-positive tumor cells in vitro. Collectively, these results establish polySia as a valid cell-surface, cancer-specific target for glycan-directed ADC and contribute to a growing body of evidence that the tumor glycocalyx is a promising target for synthetic immunotherapies. SIGNIFICANCE: These findings describe a glycan-specific antibody-drug conjugate that establishes polySia as a viable cell surface target within the tumor glycocalyx.
©2019 American Association for Cancer Research.
Conflict of interest statement
Figures


Similar articles
-
Molecular Basis of the Receptor Interactions of Polysialic Acid (polySia), polySia Mimetics, and Sulfated Polysaccharides.ChemMedChem. 2016 May 6;11(9):990-1002. doi: 10.1002/cmdc.201500609. Epub 2016 May 2. ChemMedChem. 2016. PMID: 27136597
-
Photothermal Therapy of Neuroblastoma via Polysialic Acid-Targeting Nanomissiles.Small. 2022 Nov;18(46):e2201671. doi: 10.1002/smll.202201671. Epub 2022 Sep 26. Small. 2022. PMID: 36161701
-
Polysialyltransferase: a new target in metastatic cancer.Curr Cancer Drug Targets. 2012 Oct;12(8):925-39. doi: 10.2174/156800912803251225. Curr Cancer Drug Targets. 2012. PMID: 22463390 Review.
-
A cancer-unique glycan: de-N-acetyl polysialic acid (dPSA) linked to cell surface nucleolin depends on re-expression of the fetal polysialyltransferase ST8SIA2 gene.J Exp Clin Cancer Res. 2021 Sep 20;40(1):293. doi: 10.1186/s13046-021-02099-y. J Exp Clin Cancer Res. 2021. PMID: 34544457 Free PMC article.
-
Disialic, oligosialic and polysialic acids: distribution, functions and related disease.J Biochem. 2013 Aug;154(2):115-36. doi: 10.1093/jb/mvt057. Epub 2013 Jun 20. J Biochem. 2013. PMID: 23788662 Review.
Cited by
-
Engineering affinity-matured variants of an anti-polysialic acid monoclonal antibody with superior cytotoxicity-mediating potency.bioRxiv [Preprint]. 2025 Feb 17:2025.02.12.637914. doi: 10.1101/2025.02.12.637914. bioRxiv. 2025. PMID: 40027839 Free PMC article. Preprint.
-
Antibody-Biopolymer Conjugates in Oncology: A Review.Molecules. 2023 Mar 13;28(6):2605. doi: 10.3390/molecules28062605. Molecules. 2023. PMID: 36985578 Free PMC article. Review.
-
Isolation of full-length IgG antibodies from combinatorial libraries expressed in the cytoplasm of Escherichia coli.Nat Commun. 2023 Jun 14;14(1):3514. doi: 10.1038/s41467-023-39178-x. Nat Commun. 2023. PMID: 37316535 Free PMC article.
-
Targeting Tumor Glycans for Cancer Therapy: Successes, Limitations, and Perspectives.Cancers (Basel). 2022 Jan 27;14(3):645. doi: 10.3390/cancers14030645. Cancers (Basel). 2022. PMID: 35158915 Free PMC article. Review.
-
Cell surface GRP78 regulates BACE2 via lysosome-dependent manner to maintain mesenchymal phenotype of glioma stem cells.J Exp Clin Cancer Res. 2021 Jan 7;40(1):20. doi: 10.1186/s13046-020-01807-4. J Exp Clin Cancer Res. 2021. PMID: 33413577 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

