Voltage-dependent activation in EAG channels follows a ligand-receptor rather than a mechanical-lever mechanism
- PMID: 30808709
- PMCID: PMC6484144
- DOI: 10.1074/jbc.RA119.007626
Voltage-dependent activation in EAG channels follows a ligand-receptor rather than a mechanical-lever mechanism
Abstract
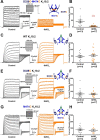
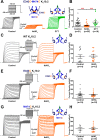
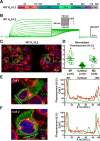
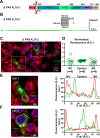
Ether-a-go-go family (EAG) channels play a major role in many physiological processes in humans, including cardiac repolarization and cell proliferation. Cryo-EM structures of two of them, KV10.1 and human ether-a-go-go-related gene (hERG or KV11.1), have revealed an original nondomain-swapped structure, suggesting that the mechanism of voltage-dependent gating of these two channels is quite different from the classical mechanical-lever model. Molecular aspects of hERG voltage-gating have been extensively studied, indicating that the S4-S5 linker (S4-S5L) acts as a ligand binding to the S6 gate (S6 C-terminal part, S6T) and stabilizes it in a closed state. Moreover, the N-terminal extremity of the channel, called N-Cap, has been suggested to interact with S4-S5L to modulate channel voltage-dependent gating, as N-Cap deletion drastically accelerates hERG channel deactivation. In this study, using COS-7 cells, site-directed mutagenesis, electrophysiological measurements, and immunofluorescence confocal microscopy, we addressed whether these two major mechanisms of voltage-dependent gating are conserved in KV10.2 channels. Using cysteine bridges and S4-S5L-mimicking peptides, we show that the ligand/receptor model is conserved in KV10.2, suggesting that this model is a hallmark of EAG channels. Truncation of the N-Cap domain, Per-Arnt-Sim (PAS) domain, or both in KV10.2 abolished the current and altered channel trafficking to the membrane, unlike for the hERG channel in which N-Cap and PAS domain truncations mainly affected channel deactivation. Our results suggest that EAG channels function via a conserved ligand/receptor model of voltage gating, but that the N-Cap and PAS domains have different roles in these channels.
Keywords: EAG channel; Kv 10.2 channel; S4-S5 linker; S6 C-terminus; allosteric regulation; biophysics; electrophysiology; ion channel; peptides; physiology; voltage dependence.
© 2019 Malak et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures







References
-
- Jiménez-Perez L., Cidad P., Álvarez-Miguel I., Santos-Hipólito A., Torres-Merino R., Alonso E., de la Fuente M. Á., López-López J. R., and Pérez-García M. T. (2016) Molecular determinants of Kv1.3 potassium channels-induced proliferation. J. Biol. Chem. 291, 3569–3580 10.1074/jbc.M115.678995 - DOI - PMC - PubMed
-
- Piron J., Choveau F. S., Amarouch M. Y., Rodriguez N., Charpentier F., Mérot J., Baró I., and Loussouarn G. (2010) KCNE1-KCNQ1 osmoregulation by interaction of phosphatidylinositol-4,5-bisphosphate with Mg2+ and polyamines. J. Physiol. 588, 3471–3483 10.1113/jphysiol.2010.195313 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

