Differential interactions of missing in metastasis and insulin receptor tyrosine kinase substrate with RAB proteins in the endocytosis of CXCR4
- PMID: 30808710
- PMCID: PMC6484128
- DOI: 10.1074/jbc.RA118.006071
Differential interactions of missing in metastasis and insulin receptor tyrosine kinase substrate with RAB proteins in the endocytosis of CXCR4
Abstract
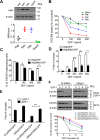
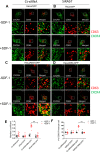
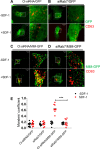
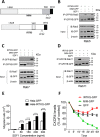
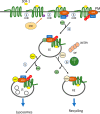
Missing in metastasis (MIM), an inverse Bin-Amphiphysin-Rvs (I-BAR) domain protein, promotes endocytosis of C-X-C chemokine receptor 4 (CXCR4) in mammalian cells. In response to the CXCR4 ligand stromal cell-derived factor 1 (SDF-1 or CXCL12), MIM associates with RAS-related GTP-binding protein 7 (RAB7) 30 min after stimulation. However, RAB7's role in MIM function remains undefined. Here we show that RNAi-mediated suppression of RAB7 expression in human HeLa cells has little effect on the binding of MIM to RAB5 and on the recruitment of CXCR4 to early endosomes but effectively abolishes MIM-mediated CXCR4 degradation, chemotactic response, and sorting into late endosomes and lysosomes. To determine whether I-BAR domain proteins interact with RAB7, we examined cells expressing insulin receptor tyrosine kinase substrate (IRTKS), an I-BAR domain protein bearing an Src homology 3 (SH3) domain. We observed that both MIM and IRTKS interact with RAB5 at an early response to SDF-1 and that IRTKS binds poorly to RAB7 but strongly to RAB11 at a later time point. Moreover, IRTKS overexpression reduced CXCR4 internalization and enhanced the chemotactic response to SDF-1. Interestingly, deletion of the SH3 domain in IRTKS abolished the IRTKS-RAB11 interaction and promoted CXCR4 degradation. Furthermore, the SH3 domain was required for selective targeting of MIM-IRTKS fusion proteins by both RAB7 and RAB11. Hence, to the best of our knowledge, our results provide first evidence that the SH3 domain is critical in the regulation of specific endocytic pathways by I-BAR domain proteins.
Keywords: C-X-C chemokine receptor type 4 (CXCR-4); RAB11; RAB7; Rab; SH3; cell signaling; chemokine; chemotaxis; endocytosis; endosome; insulin receptor tyrosine kinase substrate (IRTKS); missing in metastasis (MIM).
© 2019 Li et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures

References
-
- Saarikangas J., Mattila P. K., Varjosalo M., Bovellan M., Hakanen J., Calzada-Wack J., Tost M., Jennen L., Rathkolb B., Hans W., Horsch M., Hyvönen M. E., Perälä N., Fuchs H., Gailus-Durner V., et al. (2011) Missing-in-metastasis MIM/MTSS1 promotes actin assembly at intercellular junctions and is required for integrity of kidney epithelia. J. Cell Sci. 124, 1245–1255 10.1242/jcs.082610 - DOI - PubMed
-
- Saarikangas J., Kourdougli N., Senju Y., Chazal G., Segerstrale M., Minkeviciene R., Kuurne J., Mattila P. K., Garrett L., Hölter S. M., Becker L., Racz I., Hans W., Klopstock T., Wurst W., et al. (2015) MIM-induced membrane bending promotes dendritic spine initiation. Dev. Cell 33, 644–659 10.1016/j.devcel.2015.04.014 - DOI - PubMed
-
- Brown A. S., Meera P., Altindag B., Chopra R., Perkins E. M., Paul S., Scoles D. R., Tarapore E., Magri J., Huang H., Jackson M., Shakkottai V. G., Otis T. S., Pulst S. M., Atwood S. X., and Oro A. E. (2018) MTSS1/Src family kinase dysregulation underlies multiple inherited ataxias. Proc. Natl. Acad. Sci. U.S.A. 115, E12407–E12416 10.1073/pnas.1816177115 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

