Snapshot of an oxygen intermediate in the catalytic reaction of cytochrome c oxidase
- PMID: 30808749
- PMCID: PMC6397517
- DOI: 10.1073/pnas.1814526116
Snapshot of an oxygen intermediate in the catalytic reaction of cytochrome c oxidase
Abstract
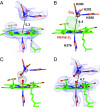
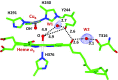
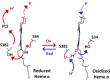
Cytochrome c oxidase (CcO) reduces dioxygen to water and harnesses the chemical energy to drive proton translocation across the inner mitochondrial membrane by an unresolved mechanism. By using time-resolved serial femtosecond crystallography, we identified a key oxygen intermediate of bovine CcO. It is assigned to the PR-intermediate, which is characterized by specific redox states of the metal centers and a distinct protein conformation. The heme a3 iron atom is in a ferryl (Fe4+ = O2-) configuration, and heme a and CuB are oxidized while CuA is reduced. A Helix-X segment is poised in an open conformational state; the heme a farnesyl sidechain is H-bonded to S382, and loop-I-II adopts a distinct structure. These data offer insights into the mechanism by which the oxygen chemistry is coupled to unidirectional proton translocation.
Keywords: X-ray free electron laser; bioenergetics; catalytic intermediates; complex IV; crystallography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Yoshikawa S, Shimada A. Reaction mechanism of cytochrome c oxidase. Chem Rev. 2015;115:1936–1989. - PubMed
-
- Belevich I, Verkhovsky MI. Molecular mechanism of proton translocation by cytochrome c oxidase. Antioxid Redox Signal. 2008;10:1–29. - PubMed
-
- Han S, Takahashi S, Rousseau DL. Time dependence of the catalytic intermediates in cytochrome c oxidase. J Biol Chem. 2000;275:1910–1919. - PubMed
-
- Morgan JE, Verkhovsky MI, Palmer G, Wikström M. Role of the PR intermediate in the reaction of cytochrome c oxidase with O2. Biochemistry. 2001;40:6882–6892. - PubMed
-
- Morgan JE, Li PM, Jang DJ, el-Sayed MA, Chan SI. Electron transfer between cytochrome a and copper A in cytochrome c oxidase: A perturbed equilibrium study. Biochemistry. 1989;28:6975–6983. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

