Human complement factor H Y402H polymorphism causes an age-related macular degeneration phenotype and lipoprotein dysregulation in mice
- PMID: 30808757
- PMCID: PMC6397537
- DOI: 10.1073/pnas.1814014116
Human complement factor H Y402H polymorphism causes an age-related macular degeneration phenotype and lipoprotein dysregulation in mice
Abstract
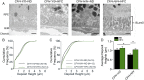
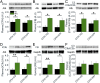
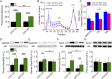
One of the strongest susceptibility genes for age-related macular degeneration (AMD) is complement factor H (CFH); however, its impact on AMD pathobiology remains unresolved. Here, the effect of the principal AMD-risk-associated CFH variant (Y402H) on the development and progression of age-dependent AMD-like pathologies was determined in vivo. Transgenic mice expressing equal amounts of the full-length normal human CFH Y402 (CFH-Y/0) or the AMD-risk associated CFH H402 (CFH-H/H) variant on a Cfh-/- background were aged to 90 weeks and switched from normal diet (ND) to a high fat, cholesterol-enriched (HFC) diet for 8 weeks. The resulting phenotype was compared with age-matched controls maintained on ND. Remarkably, an AMD-like phenotype consisting of vision loss, increased retinal pigmented epithelium (RPE) stress, and increased basal laminar deposits was detected only in aged CFH-H/H mice following the HFC diet. These changes were not observed in aged CFH-Y/0 mice or in younger (36- to 40-week-old) CFH mice of both genotypes fed either diet. Biochemical analyses of aged CFH mice after HFC diet revealed genotype-dependent changes in plasma and eyecup lipoproteins, but not complement activation, which correlated with the AMD-like phenotype in old CFH-H/H mice. Specifically, apolipoproteins B48 and A1 are elevated in the RPE/choroid of the aged CFH-H/H mice compared with age-matched control CFH-Y/0 fed a HFC diet. Hence, we demonstrate a functional consequence of the Y402H polymorphism in vivo, which promotes AMD-like pathology development and affects lipoprotein levels in aged mice. These findings support targeting lipoproteins as a viable therapeutic strategy for treating AMD.
Keywords: RPE; age-related macular degeneration; complement; genetic risk; lipoproteins.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

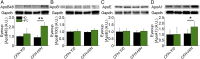
Similar articles
-
Regulation of age-related macular degeneration-like pathology by complement factor H.Proc Natl Acad Sci U S A. 2015 Jun 9;112(23):E3040-9. doi: 10.1073/pnas.1424391112. Epub 2015 May 19. Proc Natl Acad Sci U S A. 2015. PMID: 25991857 Free PMC article.
-
Cfh genotype interacts with dietary glycemic index to modulate age-related macular degeneration-like features in mice.Invest Ophthalmol Vis Sci. 2014 Jan 23;55(1):492-501. doi: 10.1167/iovs.13-12413. Invest Ophthalmol Vis Sci. 2014. PMID: 24370827 Free PMC article.
-
Bisretinoid-mediated complement activation on retinal pigment epithelial cells is dependent on complement factor H haplotype.J Biol Chem. 2014 Mar 28;289(13):9113-20. doi: 10.1074/jbc.M114.548669. Epub 2014 Feb 18. J Biol Chem. 2014. PMID: 24550392 Free PMC article.
-
Complement factor H in AMD: Bridging genetic associations and pathobiology.Prog Retin Eye Res. 2018 Jan;62:38-57. doi: 10.1016/j.preteyeres.2017.09.001. Epub 2017 Sep 18. Prog Retin Eye Res. 2018. PMID: 28928087 Free PMC article. Review.
-
[The pharmacogenomics of CFH Y402H and wet age-related macular degeneration].Zhonghua Yan Ke Za Zhi. 2017 Feb 11;53(2):144-147. doi: 10.3760/cma.j.issn.0412-4081.2017.02.014. Zhonghua Yan Ke Za Zhi. 2017. PMID: 28260367 Review. Chinese.
Cited by
-
Loss of Complement Factor H impairs antioxidant capacity and energy metabolism of human RPE cells.Sci Rep. 2020 Jun 25;10(1):10320. doi: 10.1038/s41598-020-67292-z. Sci Rep. 2020. PMID: 32587311 Free PMC article.
-
Dysfunctional Autophagy, Proteostasis, and Mitochondria as a Prelude to Age-Related Macular Degeneration.Int J Mol Sci. 2023 May 15;24(10):8763. doi: 10.3390/ijms24108763. Int J Mol Sci. 2023. PMID: 37240109 Free PMC article. Review.
-
Mice With a Combined Deficiency of Superoxide Dismutase 1 (Sod1), DJ-1 (Park7), and Parkin (Prkn) Develop Spontaneous Retinal Degeneration With Aging.Invest Ophthalmol Vis Sci. 2019 Sep 3;60(12):3740-3751. doi: 10.1167/iovs.19-27212. Invest Ophthalmol Vis Sci. 2019. PMID: 31487745 Free PMC article.
-
RPE-Directed Gene Therapy Improves Mitochondrial Function in Murine Dry AMD Models.Int J Mol Sci. 2023 Feb 14;24(4):3847. doi: 10.3390/ijms24043847. Int J Mol Sci. 2023. PMID: 36835257 Free PMC article.
-
Structural insights into the biological functions of the long pentraxin PTX3.Front Immunol. 2023 Oct 9;14:1274634. doi: 10.3389/fimmu.2023.1274634. eCollection 2023. Front Immunol. 2023. PMID: 37885881 Free PMC article. Review.
References
-
- Wong WL, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

