Paneth cell α-defensins HD-5 and HD-6 display differential degradation into active antimicrobial fragments
- PMID: 30808760
- PMCID: PMC6397583
- DOI: 10.1073/pnas.1817376116
Paneth cell α-defensins HD-5 and HD-6 display differential degradation into active antimicrobial fragments
Abstract
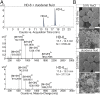
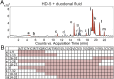
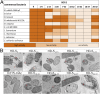
Antimicrobial peptides, in particular α-defensins expressed by Paneth cells, control microbiota composition and play a key role in intestinal barrier function and homeostasis. Dynamic conditions in the local microenvironment, such as pH and redox potential, significantly affect the antimicrobial spectrum. In contrast to oxidized peptides, some reduced defensins exhibit increased vulnerability to proteolytic degradation. In this report, we investigated the susceptibility of Paneth-cell-specific human α-defensin 5 (HD-5) and -6 (HD-6) to intestinal proteases using natural human duodenal fluid. We systematically assessed proteolytic degradation using liquid chromatography-mass spectrometry and identified several active defensin fragments capable of impacting bacterial growth of both commensal and pathogenic origins. Of note, incubation of mucus with HD-5 resulted in 255-8,000 new antimicrobial combinations. In contrast, HD-6 remained stable with consistent preserved nanonet formation. In vivo studies demonstrated proof of concept that a HD-5 fragment shifted microbiota composition (e.g., increases of Akkermansia sp.) without decreasing diversity. Our data support the concept that secretion of host peptides results in an environmentally dependent increase of antimicrobial defense by clustering in active peptide fragments. This complex clustering mechanism dramatically increases the host's ability to control pathogens and commensals. These findings broaden our understanding of host modulation of the microbiome as well as the complexity of human mucosal defense mechanisms, thus providing promising avenues to explore for drug development.
Keywords: antimicrobial peptides; host–microbiota interaction; intestinal barrier; proteolytic digestion; α-defensins.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: J. Wehkamp and D.E. are planning to apply for a patent on the HD-5 fragments.
Figures

References
-
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. - PubMed
-
- Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. - PubMed
-
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. - PubMed
-
- Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

