Specificity, versatility, and control of TGF-β family signaling
- PMID: 30808818
- PMCID: PMC6800142
- DOI: 10.1126/scisignal.aav5183
Specificity, versatility, and control of TGF-β family signaling
Abstract
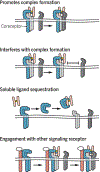
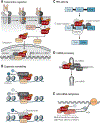
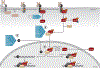
Encoded in mammalian cells by 33 genes, the transforming growth factor-β (TGF-β) family of secreted, homodimeric and heterodimeric proteins controls the differentiation of most, if not all, cell lineages and many aspects of cell and tissue physiology in multicellular eukaryotes. Deregulation of TGF-β family signaling leads to developmental anomalies and disease, whereas enhanced TGF-β signaling contributes to cancer and fibrosis. Here, we review the fundamentals of the signaling mechanisms that are initiated upon TGF-β ligand binding to its cell surface receptors and the dependence of the signaling responses on input from and cooperation with other signaling pathways. We discuss how cells exquisitely control the functional presentation and activation of heteromeric receptor complexes of transmembrane, dual-specificity kinases and, thus, define their context-dependent responsiveness to ligands. We also introduce the mechanisms through which proteins called Smads act as intracellular effectors of ligand-induced gene expression responses and show that the specificity and impressive versatility of Smad signaling depend on cross-talk from other pathways. Last, we discuss how non-Smad signaling mechanisms, initiated by distinct ligand-activated receptor complexes, complement Smad signaling and thus contribute to cellular responses.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
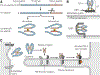
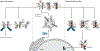

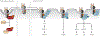
References
-
- Derynck R, Miyazono K, The Biology of the TGF-β Family. (Cold Spring Harbor Laboratory Press, New York, ed. Derynck Rik, Miyazono Kohei 2016), pp. 1164.
-
- Derynck R, Jarrett JA, Chen EY, Eaton DH, Bell JR, Assoian RK, Roberts AB, Sporn MB, Goeddel DV, Human transforming growth factor-β complementary DNA sequence and expression in normal and transformed cells. Nature 316, 701–705 (1985). - PubMed

