Integrative analysis of postharvest chilling injury in cherry tomato fruit reveals contrapuntal spatio-temporal responses to ripening and cold stress
- PMID: 30808915
- PMCID: PMC6391400
- DOI: 10.1038/s41598-019-38877-0
Integrative analysis of postharvest chilling injury in cherry tomato fruit reveals contrapuntal spatio-temporal responses to ripening and cold stress
Abstract
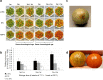
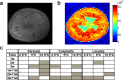
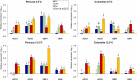
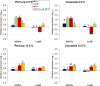
Postharvest chilling injury (PCI) reduces fruit quality and shelf-life in tomato (Solanum lycopersicum L.). PCI has been traditionally studied in the pericarp, however its development is likely heterogeneous in different fruit tissues. To gain insight into PCI's spatio-temporal development, we used postharvest biomarkers e.g. respiration and ethylene rates, ion leakage etc., to confirm the occurrence of PCI, and compared these data with molecular (gene expression), biophysical (MRI data) and biochemical parameters (Malondialdehyde (MDA) and starch content) from the pericarp or columella. Tissues were stored at control (12.5 °C) or PCI-inducing temperatures (2.5 or 5 °C) followed by rewarming at 20 °C. MRI and ion leakage revealed that cold irreversibly impairs ripening-associated membrane liquefaction; MRI also showed that the internal and external fruit tissues responded differently to cold. MDA and especially starch contents, were affected by chilling in a tissue-specific manner. The expression of the six genes studied: ACO1 and ACS2 (ripening), CBF1 (cold response), DHN, AOX1a and LoxB (stress-related) showed non-overlapping temporal and spatially-specific responses. Overall, the data highlighted the interconnectedness of fruit cold response and ripening, and showed how cold stress reconfigures the latter. They further underscored that multidimensional spatial and temporal biological studies are needed to develop effective solutions to PCI.
Conflict of interest statement
The authors declare no competing interests.
Figures


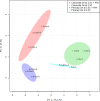
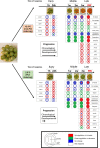
References
-
- Kader, A. Postharvest Biology and Technology: An Overview in Postharvest Technology for Horticultural Crops (ed. Kader, A.) 39–47 (UCANR, 2002).
-
- Lyons JM. Chilling injury in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1973;24:445–466. doi: 10.1146/annurev.pp.24.060173.002305. - DOI
-
- Jackman RL, Yada RY, Marangoni A, Parkin KL, Stanley DW. Chilling injury - A review of quality aspects. Journal of Food Quality. 1989;11:253–278. doi: 10.1111/j.1745-4557.1988.tb00887.x. - DOI
-
- Paul, R. E. Chilling Injury of Crops of Tropical and Subtropical Origin in Chilling injury of horticultural crops (ed. Wang, C. Y.) 17–36 (CRC Press, 1990).
-
- Parkin K, Marangoni A, Jackman R, Yada R, Stanley D. Chilling injury. A review of possible mechanisms. Journal of Food Biochemistry. 1989;13:127–153. doi: 10.1111/j.1745-4514.1989.tb00389.x. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

