Mieap-induced accumulation of lysosomes within mitochondria (MALM) regulates gastric cancer cell invasion under hypoxia by suppressing reactive oxygen species accumulation
- PMID: 30808977
- PMCID: PMC6391448
- DOI: 10.1038/s41598-019-39563-x
Mieap-induced accumulation of lysosomes within mitochondria (MALM) regulates gastric cancer cell invasion under hypoxia by suppressing reactive oxygen species accumulation
Abstract
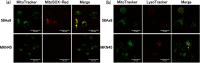
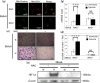
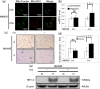
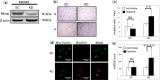
Mitochondrial quality control (MQC) protects against potentially damaging events, such as excessive generation of mitochondrial reactive oxygen species (mtROS). We investigated the contribution of the two major MQC processes, namely, mitophagy and Mieap-induced accumulation of lysosomes within mitochondria (MALM), to the response to hypoxia of two human gastric cancer (GC) cell lines. We found that hypoxia increased mtROS generation and cell invasion in 58As9, but not in MKN45, although the transcription factor hypoxia-inducible factor 1α was induced in both cell lines. Colocalisation of lysosomes with mitochondria was found only in hypoxic MKN45 cells, suggesting that hypoxia-induced MQC functions normally in MKN45 but may be impaired in 58As9. Hypoxia did not lead to decreased mitochondrial mass or DNA or altered appearance of autophagosomes, as judged by electron microscopy, suggesting that mitophagy was not induced in either cell line. However, western blot analysis revealed the presence of the MALM-associated proteins Mieap, BNIP3 and BNIP3L, and the lysosomal protein cathepsin D in the mitochondrial fraction of MKN45 cells under hypoxia. Finally, Mieap knockdown in MKN45 cells resulted in increased mtROS accumulation and cell invasion under hypoxia. Our results suggest that hypoxia-induced MALM suppresses GC cell invasion by preventing mtROS generation.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Georgieva E, et al. Mitochondrial dysfunction and redox imbalance as a diagnostic marker of “Free Radical Diseases”. ANTICANCER RES. 2017;37:5375–5381. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

