Targeted and immune therapies for hepatocellular carcinoma: Predictions for 2019 and beyond
- PMID: 30809080
- PMCID: PMC6385008
- DOI: 10.3748/wjg.v25.i7.789
Targeted and immune therapies for hepatocellular carcinoma: Predictions for 2019 and beyond
Abstract
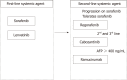
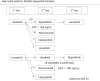
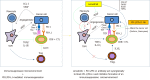
Systemic therapy for hepatocellular carcinoma (HCC) has markedly advanced since the survival benefit of a molecular targeted agent, sorafenib, were demonstrated in the SHARP and Asia Pacific trials in 2007. Treatment options for patients with advanced HCC increased by sorafenib, and long-term survival for patients with advanced stage HCC has become possible to some extent. However, development of a more potent first-line novel molecular targeted agent replacing sorafenib and a potent second-line agent after disease progression on or intolerant to sorafenib has been warranted because sorafenib lacks tumor shrinking/necrotizing effects and induces relatively severe adverse events such as hand foot skin reaction. Many agents in the 1st line and 2nd line setting were attempted to develop between 2007 and 2016, but all of these clinical trials failed. On the other hand, clinical trials of 4 agents (regorafenib, lenvatinib, cabozantinib, and ramucirumab) succeeded in succession in 2017 and 2018, and their use in clinical practice is possible (regorafenib and lenvatinib) or underway (cabozantinib and ramucirumab). Furthermore, all of 5 clinical trials of combination therapy with transcatheter chemoembolization (TACE) plus a molecular targeted agent failed to date, however, the combination of TACE and sorafenib (TACTICS trials) was reported to be successful and presented at ASCO in 2018. Phase 3 clinical trials of immune checkpoint inhibitors and a combination therapy of immune checkpoint inhibitors and molecular targeted agents are also ongoing, which suggests treatment paradigm of HCC in all stages from early, intermediate and advanced stage, is expected to be changed drastically in the very near future.
Keywords: Hepatocellular carcinoma; Immune checkpoint inhibitor; Lenvatinib; Molecular targeted agent; Sorafenib.
Conflict of interest statement
Conflict-of-interest statement: Masatoshi Kudo received lecture fees from Bayer, Eisai, MSD, and Ajinomoto, research grants from Chugai, Otsuka, Takeda, Taiho, Sumitomo Dainippon, Daiichi Sankyo, MSD, Eisai, Bayer, AbbVie, Medico’s Hirata, Astellas Pharma, and Bristol-Myers Squibb, and advisory consulting fees from Kowa, MSD, Bristol-Myers Squibb, Bayer, Chugai, Taiho, Eisai, and Ono Pharmaceutical.
Figures


Similar articles
-
Current Standard and Future Perspectives in Non-Surgical Therapy for Hepatocellular Carcinoma.Digestion. 2017;96(1):1-4. doi: 10.1159/000464282. Epub 2017 Jun 13. Digestion. 2017. PMID: 28605745 Free PMC article. Review.
-
Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma.J Exp Clin Cancer Res. 2019 Nov 4;38(1):447. doi: 10.1186/s13046-019-1412-8. J Exp Clin Cancer Res. 2019. PMID: 31684985 Free PMC article. Review.
-
Strategies to Improve the Antitumor Effect of Immunotherapy for Hepatocellular Carcinoma.Front Immunol. 2021 Nov 26;12:783236. doi: 10.3389/fimmu.2021.783236. eCollection 2021. Front Immunol. 2021. PMID: 34899747 Free PMC article. Review.
-
Optimizing Survival and the Changing Landscape of Targeted Therapy for Intermediate and Advanced Hepatocellular Carcinoma: A Systematic Review.J Natl Cancer Inst. 2021 Feb 1;113(2):123-136. doi: 10.1093/jnci/djaa119. J Natl Cancer Inst. 2021. PMID: 32898239 Free PMC article.
-
Review article: new therapeutic interventions for advanced hepatocellular carcinoma.Aliment Pharmacol Ther. 2020 Jan;51(1):78-89. doi: 10.1111/apt.15573. Epub 2019 Nov 20. Aliment Pharmacol Ther. 2020. PMID: 31747082 Review.
Cited by
-
The Complexity of the Tumor Microenvironment in Hepatocellular Carcinoma and Emerging Therapeutic Developments.J Clin Med. 2023 Dec 2;12(23):7469. doi: 10.3390/jcm12237469. J Clin Med. 2023. PMID: 38068521 Free PMC article. Review.
-
New insights into checkpoint inhibitor immunotherapy and its combined therapies in hepatocellular carcinoma: from mechanisms to clinical trials.Int J Biol Sci. 2022 Mar 28;18(7):2775-2794. doi: 10.7150/ijbs.70691. eCollection 2022. Int J Biol Sci. 2022. PMID: 35541908 Free PMC article. Review.
-
Treatment with a new benzimidazole derivative bearing a pyrrolidine side chain overcomes sorafenib resistance in hepatocellular carcinoma.Sci Rep. 2019 Nov 21;9(1):17259. doi: 10.1038/s41598-019-53863-2. Sci Rep. 2019. PMID: 31754201 Free PMC article.
-
Immune cell therapy for hepatocellular carcinoma.J Hematol Oncol. 2019 May 29;12(1):52. doi: 10.1186/s13045-019-0742-5. J Hematol Oncol. 2019. PMID: 31142330 Free PMC article. Review.
-
Current statuses of molecular targeted and immune checkpoint therapies in hepatocellular carcinoma.Am J Cancer Res. 2020 May 1;10(5):1522-1533. eCollection 2020. Am J Cancer Res. 2020. PMID: 32509395 Free PMC article.
References
-
- Japan Soceity of Hepatology. Clinical Practice Guidelines for Hepatocellular Carcinoma. Tokyo: Kanehara Co; 2017. p. (in Japanese).
-
- Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol Int. 2017;11:317–370. - PMC - PubMed
-
- European Association for the Study of the Liver; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. - PubMed
-
- Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. - PubMed
-
- Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition) Liver Cancer. 2018;7:235–260. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

