Activated Wnt signaling promotes growth and progression of AFP-producing gastric cancer in preclinical models
- PMID: 30809100
- PMCID: PMC6376882
- DOI: 10.2147/CMAR.S187219
Activated Wnt signaling promotes growth and progression of AFP-producing gastric cancer in preclinical models
Abstract
Background: Characterized by elevated AFP levels in serum, AFP-producing gastric cancer (APGC) is a very special type of gastric cancer (GC) that is difficult to treat and has poor prognosis. However, little is known about the role of AFP in GC, which was investigated in this study with in vitro and in vivo experiments.
Methods: APGC cells were established with lentivirus infection and validated by PCR assay and ELISA in HCG27 and AGS cells. Cell growth, migration, and invasion were determined by CCK8, transwell assays, and animal experiments. RNA sequencing, Western blot, dual-luciferase-reporter assays, and RNA interference were employed to understand mechanisms underlying AFP activity, followed by therapeutic investigations for APGC.
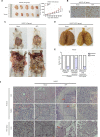
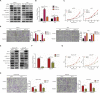
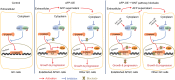
Results: APGC cells featured significantly increased AFP levels in cellular supernatants. AFP potentiated growth and aggression in GC cell lines and their derived xenografts. Wnt-signaling activation was responsible for AFP function, indicated by decreased Axin 1 and pGSK3β, followed by cascade activation of β-catenin, downstream transcription factors TCF1/TCF7, and the target gene - c-Myc. Wnt-signaling blockade by Axin 1 rescue or pathway inhibitor XAV939 reversed AFP function, suggesting the potential therapeutic value of APGC.
Conclusion: AFP played a critical role in APGC through activating Wnt signaling, and targeting Wnt pathways might be a promising strategy against APGC.
Keywords: AFP; AFP-producing gastric cancer; APGC; Axin 1; Wnt signaling; Wnt-signaling inhibitor; alpha-fetoprotein.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Angiogenesis of AFP producing gastric carcinoma: correlation with frequent liver metastasis and its inhibition by anti-AFP antibody.Oncol Rep. 2004 Apr;11(4):809-13. Oncol Rep. 2004. PMID: 15010877
-
Biological aggressiveness of alpha-fetoprotein (AFP)-positive gastric cancer.Hepatogastroenterology. 2006 May-Jun;53(69):338-41. Hepatogastroenterology. 2006. PMID: 16795967
-
MiRNA-27a promotes the proliferation and invasion of human gastric cancer MGC803 cells by targeting SFRP1 via Wnt/β-catenin signaling pathway.Am J Cancer Res. 2017 Mar 1;7(3):405-416. eCollection 2017. Am J Cancer Res. 2017. PMID: 28401000 Free PMC article.
-
Aberrantly expressed miR-188-5p promotes gastric cancer metastasis by activating Wnt/β-catenin signaling.BMC Cancer. 2019 May 28;19(1):505. doi: 10.1186/s12885-019-5731-0. BMC Cancer. 2019. PMID: 31138169 Free PMC article.
-
Ubiquitin-like modifier activating enzyme 2 promotes cell migration and invasion through Wnt/β-catenin signaling in gastric cancer.World J Gastroenterol. 2018 Nov 14;24(42):4773-4786. doi: 10.3748/wjg.v24.i42.4773. World J Gastroenterol. 2018. PMID: 30479464 Free PMC article.
Cited by
-
Predictive biomarkers in the era of immunotherapy for gastric cancer: current achievements and future perspectives.Front Immunol. 2025 May 14;16:1599908. doi: 10.3389/fimmu.2025.1599908. eCollection 2025. Front Immunol. 2025. PMID: 40438098 Free PMC article. Review.
-
Clinical prognosis evaluation of alpha-fetoprotein-positive gastric cancer: comprehensive analysis and development of a novel nomogram for survival prediction.Front Oncol. 2025 May 23;15:1598337. doi: 10.3389/fonc.2025.1598337. eCollection 2025. Front Oncol. 2025. PMID: 40485723 Free PMC article.
-
Both the serum AFP test and AFP/GPC3/SALL4 immunohistochemistry are beneficial for predicting the prognosis of gastric adenocarcinoma.BMC Gastroenterol. 2021 Oct 27;21(1):408. doi: 10.1186/s12876-021-01986-0. BMC Gastroenterol. 2021. PMID: 34706681 Free PMC article.
-
Upregulation of family with sequence similarity 83 member D expression enhances cell proliferation and motility via activation of Wnt/β-catenin signaling and predicts poor prognosis in gastric cancer.Cancer Manag Res. 2019 Jul 22;11:6775-6791. doi: 10.2147/CMAR.S203082. eCollection 2019. Cancer Manag Res. 2019. PMID: 31413630 Free PMC article.
-
Wnt Signaling in Cancer Metabolism and Immunity.Cancers (Basel). 2019 Jun 28;11(7):904. doi: 10.3390/cancers11070904. Cancers (Basel). 2019. PMID: 31261718 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

