Randomized clinical trials with run-in periods: frequency, characteristics and reporting
- PMID: 30809104
- PMCID: PMC6377048
- DOI: 10.2147/CLEP.S188752
Randomized clinical trials with run-in periods: frequency, characteristics and reporting
Abstract
Background: Run-in periods are occasionally used in randomized clinical trials to exclude patients after inclusion, but before randomization. In theory, run-in periods increase the probability of detecting a potential treatment effect, at the cost of possibly affecting external and internal validity. Adequate reporting of exclusions during the run-in period is a prerequisite for judging the risk of compromised validity. Our study aims were to assess the proportion of randomized clinical trials with run-in periods, to characterize such trials and the types of run-in periods and to assess their reporting.
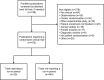
Materials and methods: This was an observational study of 470 PubMed-indexed randomized controlled trial publications from 2014. We compared trials with and without run-in periods, described the types of run-in periods and evaluated the completeness of their reporting by noting whether publications stated the number of excluded patients, reasons for exclusion and baseline characteristics of the excluded patients.
Results: Twenty-five trials reported a run-in period (5%). These were larger than other trials (median number of randomized patients 217 vs 90, P=0.01) and more commonly industry trials (11% vs 3%, P<0.01). The run-in procedures varied in design and purpose. In 23 out of 25 trials (88%), the run-in period was incompletely reported, mostly due to missing baseline characteristics.
Conclusion: Approximately 1 in 20 trials used run-in periods, though much more frequently in industry trials. Reporting of the run-in period was often incomplete, precluding a meaningful assessment of the impact of the run-in period on the validity of trial results. We suggest that current trials with run-in periods are interpreted with caution and that updates of reporting guidelines for randomized trials address the issue.
Keywords: enrichment design; lead-in periods; research methodology; run-in periods; single-blind placebo; washout periods.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Pablos-Méndez A, Barr RG, Shea S. Run-in periods in randomized trials: implications for the application of results in clinical practice. JAMA. 1998;279(3):222–225. - PubMed
-
- Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH) Draft guidance for industry: enrichment strategies for clinical trials to support approval of human drugs and biological products. [Accessed April 9, 2017]. Available from: http://www.fda.gov/downloads/drugs/guid-ancecomplianceregulatoryinformat.... Published December 2012.
-
- Hale M, Khan A, Kutch M, Li S. Once-daily OROS hydromorphone ER compared with placebo in opioid-tolerant patients with chronic low back pain. Curr Med Res Opin. 2010;26(6):1505–1518. - PubMed
-
- Lang JM, Buring JE, Rosner B, Cook N, Hennekens CH. Estimating the effect of the run-in on the power of the physicians’ health study. Stat Med. 1991;10(10):1585–1593. - PubMed
LinkOut - more resources
Full Text Sources
Medical

