Noxious Iron-Calcium Connections in Neurodegeneration
- PMID: 30809110
- PMCID: PMC6379295
- DOI: 10.3389/fnins.2019.00048
Noxious Iron-Calcium Connections in Neurodegeneration
Abstract
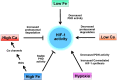
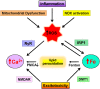
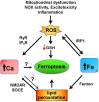
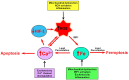
Iron and calcium share the common feature of being essential for normal neuronal function. Iron is required for mitochondrial function, synaptic plasticity, and the development of cognitive functions whereas cellular calcium signals mediate neurotransmitter exocytosis, axonal growth and synaptic plasticity, and control the expression of genes involved in learning and memory processes. Recent studies have revealed that cellular iron stimulates calcium signaling, leading to downstream activation of kinase cascades engaged in synaptic plasticity. The relationship between calcium and iron is Janus-faced, however. While under physiological conditions iron-mediated reactive oxygen species generation boosts normal calcium-dependent signaling pathways, excessive iron levels promote oxidative stress leading to the upsurge of unrestrained calcium signals that damage mitochondrial function, among other downstream targets. Similarly, increases in mitochondrial calcium to non-physiological levels result in mitochondrial dysfunction and a predicted loss of iron homeostasis. Hence, if uncontrolled, the iron/calcium self-feeding cycle becomes deleterious to neuronal function, leading eventually to neuronal death. Here, we review the multiple cell-damaging responses generated by the unregulated iron/calcium self-feeding cycle, such as excitotoxicity, free radical-mediated lipid peroxidation, and the oxidative modification of crucial components of iron and calcium homeostasis/signaling: the iron transporter DMT1, plasma membrane, and intracellular calcium channels and pumps. We discuss also how iron-induced dysregulation of mitochondrial calcium contributes to the generation of neurodegenerative conditions, including Alzheimer's disease (AD) and Parkinson's disease (PD).
Keywords: HIF-1; Nrf-2; ferroptosis; inflammation; mitochondria; neurodegenerative diseases; reactive oxygen species.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

