Sex Differences in Estradiol Secretion by Trigeminal Brainstem Neurons
- PMID: 30809134
- PMCID: PMC6379465
- DOI: 10.3389/fnint.2019.00003
Sex Differences in Estradiol Secretion by Trigeminal Brainstem Neurons
Abstract
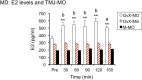
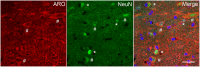
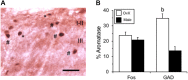
Estrogen status is a significant risk factor in the development of temporomandibular joint disorders (TMD). Classically, estrogen status is thought to derive mainly from ovarian sources; however, it is well known that estradiol (E2) also is synthesized by neurons in the brain. This study tested the hypothesis that E2 is produced by neurons in trigeminal subnucleus caudalis (Vc), the principal site of termination for sensory afferents that supply the temporomandibular joint (TMJ), to modify evoked responses in a model of TMJ nociception in male and female rats. Intra-TMJ injection of the small fiber excitant, allyl isothiocyanate (AIC), increased the levels of E2 collected from microdialysis probes sites at Vc of ovariectomized (OvX) female rats, ipsilateral to the stimulus, whereas males displayed no change. Dialysate levels of E2 collected from probe sites in the contralateral Vc or cerebellum in OvX rats were not affected by TMJ stimulation. Reverse dialysis of anastrozole, an aromatase (ARO) inhibitor, via the probe reduced perfusate levels of E2 in Vc. Systemic administration of letrozole, a non-steroid ARO inhibitor, for 4 days prevented TMJ-evoked increases in masseter muscle electromyography (MMemg) activity. ARO-positive neurons were distributed mainly in superficial laminae (I-III) at Vc and cell counts revealed no significant difference between OvX and male rats. Intra-TMJ injection of AIC revealed similar numbers of ARO/Fos dual-labeled neurons in OvX and male rats. By contrast, the percentage of ARO neurons co-labeled for glutamic acid decarboxylase (GAD), the biosynthetic enzyme for GABA, was greater in OvX (35%) than male rats (14%). Few ARO-positive neurons were co-labeled for estrogen receptor alpha. These data indicate that E2 is secreted continuously by Vc neurons and that acute stimulation of TMJ nociceptors evokes further secretion in a sex-dependent manner. Reduced TMJ-evoked MMemg activity after ARO inhibition suggests that locally produced E2 by Vc neurons acts via paracrine mechanisms to modify TMJ nociception in female rats.
Keywords: animal models; aromatase; nociception; temporomandibular disorders; trigeminal nucleus caudalis.
Figures




Similar articles
-
Local group I mGluR antagonists reduce TMJ-evoked activity of trigeminal subnucleus caudalis neurons in female rats.Neuroscience. 2015 Jul 23;299:125-33. doi: 10.1016/j.neuroscience.2015.04.051. Epub 2015 Apr 29. Neuroscience. 2015. PMID: 25934040
-
Morphine modulation of temporomandibular joint-responsive units in superficial laminae at the spinomedullary junction in female rats depends on estrogen status.Eur J Neurosci. 2008 Nov;28(10):2065-74. doi: 10.1111/j.1460-9568.2008.06488.x. Eur J Neurosci. 2008. PMID: 19046387
-
Differential effects of estradiol on encoding properties of TMJ units in laminae I and V at the spinomedullary junction in female rats.J Neurophysiol. 2007 Dec;98(6):3242-53. doi: 10.1152/jn.00677.2007. Epub 2007 Oct 10. J Neurophysiol. 2007. PMID: 17928557
-
GABAergic influence on temporomandibular joint-responsive spinomedullary neurons depends on estrogen status.Neuroscience. 2014 Feb 14;259:53-62. doi: 10.1016/j.neuroscience.2013.11.053. Epub 2013 Dec 4. Neuroscience. 2014. PMID: 24316475 Free PMC article.
-
The effects of estrogen on temporomandibular joint pain as influenced by trigeminal caudalis neurons.J Oral Sci. 2020 Mar 28;62(2):150-155. doi: 10.2334/josnusd.19-0405. Epub 2020 Mar 4. J Oral Sci. 2020. PMID: 32132330 Review.
Cited by
-
Aromatase in the Human Brain.Androg Clin Res Ther. 2021 Dec 23;2(1):189-202. doi: 10.1089/andro.2021.0007. eCollection 2021. Androg Clin Res Ther. 2021. PMID: 35024691 Free PMC article. Review.
-
Role of Connexin 43 in an Inflammatory Model for TMJ Hyperalgesia.Front Pain Res (Lausanne). 2021 Aug 3;2:715871. doi: 10.3389/fpain.2021.715871. eCollection 2021. Front Pain Res (Lausanne). 2021. PMID: 35295418 Free PMC article.
-
A neural circuit perspective on brain aromatase.Front Neuroendocrinol. 2022 Apr;65:100973. doi: 10.1016/j.yfrne.2021.100973. Epub 2021 Dec 21. Front Neuroendocrinol. 2022. PMID: 34942232 Free PMC article. Review.
-
Knowledge and awareness about temporomandibular disorder among dentists in India: Questionnaire study and review.J Indian Prosthodont Soc. 2024 Jul 1;24(3):284-291. doi: 10.4103/jips.jips_573_23. Epub 2024 Jul 1. J Indian Prosthodont Soc. 2024. PMID: 38946513 Free PMC article. Review.
-
P2Y2 Receptors Mediate Masseter Muscle Mechanical Hypersensitivity in Rats.J Pain Res. 2020 Jun 3;13:1323-1333. doi: 10.2147/JPR.S239831. eCollection 2020. J Pain Res. 2020. PMID: 32581574 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

