Chromatic Pupillometry Methods for Assessing Photoreceptor Health in Retinal and Optic Nerve Diseases
- PMID: 30809186
- PMCID: PMC6379484
- DOI: 10.3389/fneur.2019.00076
Chromatic Pupillometry Methods for Assessing Photoreceptor Health in Retinal and Optic Nerve Diseases
Abstract
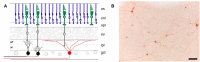
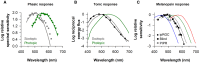
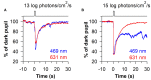
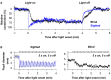
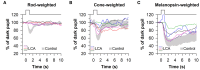
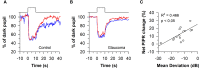
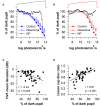
The pupillary light reflex is mediated by melanopsin-containing intrinsically-photosensitive retinal ganglion cells (ipRGCs), which also receive input from rods and cones. Melanopsin-dependent pupillary light responses are short-wavelength sensitive, have a higher threshold of activation, and are much slower to activate and de-activate compared with rod/cone-mediated responses. Given that rod/cone photoreceptors and melanopsin differ in their response properties, light stimuli can be designed to stimulate preferentially each of the different photoreceptor types, providing a read-out of their function. This has given rise to chromatic pupillometry methods that aim to assess the health of outer retinal photoreceptors and ipRGCs by measuring pupillary responses to blue or red light stimuli. Here, we review different types of chromatic pupillometry protocols that have been tested in patients with retinal or optic nerve disease, including approaches that use short-duration light exposures or continuous exposure to light. Across different protocols, patients with outer retinal disease (e.g., retinitis pigmentosa or Leber congenital amaurosis) show reduced or absent pupillary responses to dim blue-light stimuli used to assess rod function, and reduced responses to moderately-bright red-light stimuli used to assess cone function. By comparison, patients with optic nerve disease (e.g., glaucoma or ischemic optic neuropathy, but not mitochondrial disease) show impaired pupillary responses during continuous exposure to bright blue-light stimuli, and a reduced post-illumination pupillary response after light offset, used to assess melanopsin function. These proof-of-concept studies demonstrate that chromatic pupillometry methods can be used to assess damage to rod/cone photoreceptors and ipRGCs. In future studies, it will be important to determine whether chromatic pupillometry methods can be used for screening and early detection of retinal and optic nerve diseases. Such methods may also prove useful for objectively evaluating the degree of recovery to ipRGC function in blind patients who undergo gene therapy or other treatments to restore vision.
Keywords: blind; blue light; glaucoma; melanopsin; optic nerve; pupillary light reflex; pupillometry; retina.
Figures


Similar articles
-
Assessment of Rod, Cone, and Intrinsically Photosensitive Retinal Ganglion Cell Contributions to the Canine Chromatic Pupillary Response.Invest Ophthalmol Vis Sci. 2017 Jan 1;58(1):65-78. doi: 10.1167/iovs.16-19865. Invest Ophthalmol Vis Sci. 2017. PMID: 28061512 Free PMC article.
-
Chromatic pupillometry for evaluating melanopsin retinal ganglion cell function in Alzheimer's disease and other neurodegenerative disorders: a review.Front Psychol. 2024 Jan 8;14:1295129. doi: 10.3389/fpsyg.2023.1295129. eCollection 2023. Front Psychol. 2024. PMID: 38259552 Free PMC article. Review.
-
Pupillometric evaluation of the melanopsin containing retinal ganglion cells in mitochondrial and non-mitochondrial optic neuropathies.Mitochondrion. 2017 Sep;36:124-129. doi: 10.1016/j.mito.2017.07.003. Epub 2017 Jul 14. Mitochondrion. 2017. PMID: 28716667 Review.
-
Binocular head-mounted chromatic pupillometry can detect structural and functional loss in glaucoma.Front Neurosci. 2023 Jun 29;17:1187619. doi: 10.3389/fnins.2023.1187619. eCollection 2023. Front Neurosci. 2023. PMID: 37456990 Free PMC article.
-
Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans.J Neurosci. 2012 Oct 10;32(41):14242-53. doi: 10.1523/JNEUROSCI.1321-12.2012. J Neurosci. 2012. PMID: 23055493 Free PMC article. Clinical Trial.
Cited by
-
Prediction accuracy of L- and M-cone based human pupil light models.Sci Rep. 2020 Jul 3;10(1):10988. doi: 10.1038/s41598-020-67593-3. Sci Rep. 2020. PMID: 32620793 Free PMC article.
-
Resveratrol acts via the mitogen-activated protein kinase (MAPK) pathway to protect retinal ganglion cells from apoptosis induced by hydrogen peroxide.Bioengineered. 2021 Dec;12(1):4878-4886. doi: 10.1080/21655979.2021.1954742. Bioengineered. 2021. PMID: 34374636 Free PMC article.
-
Handheld chromatic pupillometry can accurately and rapidly reveal functional loss in glaucoma.Br J Ophthalmol. 2023 May;107(5):663-670. doi: 10.1136/bjophthalmol-2021-319938. Epub 2021 Dec 1. Br J Ophthalmol. 2023. PMID: 34853018 Free PMC article.
-
Chromatic Pupillometry as a Putative Screening Tool for Heritable Retinal Disease in Rhesus Macaques.Transl Vis Sci Technol. 2023 Jun 1;12(6):13. doi: 10.1167/tvst.12.6.13. Transl Vis Sci Technol. 2023. PMID: 38752621 Free PMC article.
-
From pre-processing to advanced dynamic modeling of pupil data.Behav Res Methods. 2024 Mar;56(3):1376-1412. doi: 10.3758/s13428-023-02098-1. Epub 2023 Jun 22. Behav Res Methods. 2024. PMID: 37351785 Free PMC article.
References
-
- Laurens H. Studies on the relative physiologial value of spectral lights. Am J Physiol Legacy Content (1923) 64:97–119. 10.1152/ajplegacy.1923.64.1.97 - DOI
-
- Wagman IH, Gullberg JE. The relationship between monochromatic light and pupil diameter. The low intensity visibility curve as measured by pupillary measurements. Am J Physiol Legacy Content (1942) 137:769–78. 10.1152/ajplegacy.1942.137.4.769 - DOI
Publication types
LinkOut - more resources
Full Text Sources

